H\(_2\)SO\(_4\)
C\(_2\)H\(_5\)OH → C\(_2\)H\(_4\)
170\(^0\)C
The reaction above illustrates
- A. oxidation
- B. esterification
- C. dehydration
- D. polymerization
The IUPAC Nomenclature of CH\(_3\)CH\(_2\)C(CH\(_3\))=C(CH\(_3\))\(_2\) for the compound is
- A. 2,3-dimethylpentene
- B. 2,3-dimethylpent-2-ene
- C. 3,4-dimethylpent-3-ene
- D. 3,4-dimethylpentene
Esterification reaction is analogous to
- A. oxidation reaction
- B. neutralization reaction
- C. condensation reaction
- D. hydrolysis reaction
When the subsidiary quantum numbers (l) equals 1, the shape of the orbital is
- A. spherical
- B. dumb-bell
- C. flat
- D. round
What is the vapour density of 560cm\(^3\) of a gas that weighs 0.4g at s.t.p?
[Molar Volume of gas at s.t.p = 22.4 dm\(^3\) ]
- A. 6.0
- B. 8.0
- C. 16.0
- D. 32.0
Boyle’s law can be expressed mathematically as
- A. PV = K
- B. PV = RT
- C. V =KT
- D. \(\frac{V}{T}\) = K
An example of a physical change is
- A. exposing sodium metal to air
- B. boiling of water
- C. dissolving calcium metal in water
- D. burning of kerosene
The percentage of carbon(IV) oxide in air is
- A. 0.01
- B. 0.02
- C. 0.05
- D. 0.03
The shape of ammonia molecule is
- A. V-Shaped or bent
- B. Tetrahedral
- C. co-plannar
- D. trigonal
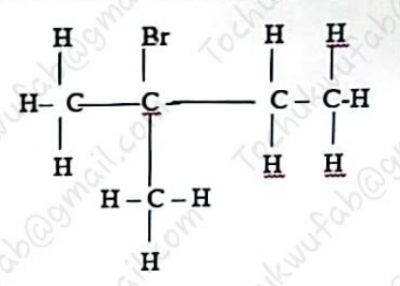
The IUPAC name of the compound above is
- A. 3-methyl, 3-bromo butane
- B. 2-methyl, 2-bromo butane
- C. 2-bromo, 2-methyl butane
- D. 3-bromo, 3-methyl butane
Heat of solution involves two steps that is accompanied by heat change. The energies involved in this steps are
- A. lattice energy and hydration energy
- B. lattice energy and ionic energy
- C. Heat energy and lattice energy
- D. hydration energy and heat energy
The amount of water a substance chemically combined with is called water of
- A. dehydration
- B. solution
- C. hydration
- D. crystallization
An example of a compound that is acidic in solution is
- A. H\(_3\)PO\(_4\)
- B. NH\(_4\)Cl
- C. Na\(_2\)CO\(_3\)
- D. MgCl\(_2\)
Biodegradable pollutants are not safe in water systems because they can cause
- A. air pollution
- B. soil degradation
- C. ill health
- D. greenhouse effect
An example of highly unsaturated hydrocarbon is
- A. C\(_2\)H\(_2\)
- B. CH\(_4\)
- C. C\(_2\)H\(_4\)
- D. C\(_4\)H\(_{10}\)
Scandium is not regarded as a transition metal because its ion has
- A. no electron in the d-orbital
- B. a partially filled d-orbitals
- C. a completely filled 4S orbital
- D. a partially filled 4S orbital
If a stable neutral atom has a mass number of 31, the number of electrons and neutrons respectively are
- A. 15 and 16
- B. 16 and 31
- C. 15 and 31
- D. 16 and 15
H\(_2\)S\(_{(g)}\) + Cl\(_2\)\(_{(g)}\) → 2HCl\(_{(g)}\) + S\(_{(s)}\)
What is the change in oxidation state of sulphur from reactant to product?
- A. -3 to -1
- B. -3 to 1
- C. -2 to 0
- D. -1 to 0
The product formed when ethyne is passed through a hot tube containing finely divided iron is
- A. polymeric
- B. saturated
- C. hydrated
- D. aromatic
An example of a physical change is
- A. slaking of lime
- B. liquefaction of liquids
- C. dissolution of limestone in acid
- D. rusting of iron
25.0g of potassium chloride were dissolved in 80g of distilled water at 30\(^0\)C. Calculate the solubility of the solute in mol dm\(^3\). [K =39, Cl = 35.5]
- A. 3.9 moldm\(^3\)
- B. 4.6 moldm\(^3\)
- C. 4.0 moldm\(^3\)
- D. 4.2 moldm\(^3\)


