The size (diameter) of five elements are in the order R < T < W < X < Y, Y being the largest. If each atom has an electron situated on its circumference, and neglecting other factors, which of the atoms will lose its electron most reluctantly?
- A. R
- B. T
- C. W
- D. X
- E. Y
Five compounds R,T,W,X and Y form the following compounds: a basic hydride R.H, an acidic hydride YH, atmospheric oxide W2O3, XO2. which of the elements is an alkali
- A. R
- B. T
- C. W
- D. X
- E. Y
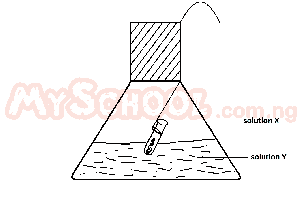
An experiment test of the Law of conservation of Mass is illustrated in the diagram. In practice, the flask is weighed before and after reaction between solutions X and Y. Which of the following pairs of solutions will be unsuitable for the experiments
- A. X = hydrochloric acid; Y = silver nitrate
- B. X = barium chloride; Y = dilute sulphuric acid
- C. X = hydrochloric acid; Y = sodium hydroxide
- D. X = hydrochloric acid; Y = lead nitrate
- E. X = hydrochloric acid; Y = sodium carbonate
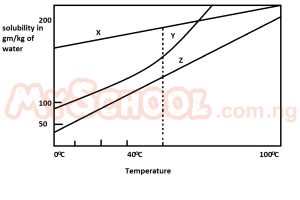
Consider the solubility curves of three salts X, Y and Z given in the diagram. If each solution of the salt contains 200 g, and is heated to 100oC, which solution or solutions will deposit 100 g of the solute when suddenly cooled to 0oC?
- A. X only
- B. Y only
- C. Z only
- D. X and Z
- E. Y and Z
A solid X when heated gives off a brown gas. If X is soluble in excess sodium hydroxide solution but insoluble in excess ammonium hydroxide solution, then X is
- A. basic lead carbonate
- B. lead (II) nitrate
- C. sodium carbonate
- D. zinc nitrate
- E. sodium nitrate
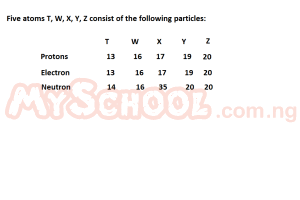
Which of the five atoms can be described by the following properties: Relative atomic mass is greater than 30 but less than 40, it has odd atomic number and forms a unipositive ion in solution?
- A. T
- B. W
- C. X
- D. Y
- E. Z
Which of the following substances would you see as an indicator in the titration of sodium carbonate solution against hydrochloric acid (complete neutralisation)?
- A. Litmus paper
- B. Phenolphthalein
- C. Methyl orange
- D. Universal indicator
- E. None of these
You are provided with five gas jars containing SO2, CO2, H2, CO and NO respectively. Select a test from A to E which will identify ANY ONE of the gases completely
- A. pass each gas into lime water
- B. pass each gas into water and test with litmus paper
- C. pass each gas into concentrated sulphuric acid
- D. expose each gas to atmospheric air
- E. expose each gas to fumes of hydrogen chloride
A gas jar was inverted over burning yellow phosphorus floating over water in a beaker. After burning, the water level was found to rise in the gas jar. The water level rises because
- A. pressure inside the gas jar is greater than pressure outside it
- B. the air in the gas jar had been used up by burning
- C. oxygen in the gas jar had been used up by burning
- D. nitrogen in the gas jar had been used up by burning
- E. the temperature in the jar had risen considerably
In titration involving sodium hydroxide and dilute hydrochloric acid, where would you place the base?
- A. in the beaker
- B. in the conical flask
- C. in a burette
- D. in the standard flask
- E. in the measuring cylinder
By means of filtration, one component can be obtained pure from an aqueous mixture of sodium chloride and?
- A. potassium nitrate
- B. sand
- C. lead nitrate
- D. sugar (glucose)
- E. starch
Which of the following changes is physical?
- A. Adding iron filing to aerated water
- B. Adding sodium metal to water
- C. Cooling a solution of Iron (II) sulphate to obtain the hydrated salt
- D. Cooling water to obtain ice
- E. Burning the domestic gas (Utilgas) for cooking
If hydrogen sulphide gas is passed into a solution of a pure iron chloride, a yellow deposit appears. If the deposit is filtered, a pale green solution is left behind. The pale green solution is
- A. dilute sulphuric acid
- B. dilute hydrochloric acid
- C. unreacted hydrogen sulphide in water
- D. iron (III) chloride
- E. Iron (II) chloride
Consider the following equation H2O + 2Fe2+ + Cl2 ↔ 2Fe3+ + 2Cl- + H2O. Which behaves as an oxidizing agent?
- A. Fe2+
- B. Cl2
- C. Fe3+
- D. Cl-
- E. H2O
Two gas cylinders contain ethylene (ethene) and acetylene respectively. One test which can be used to distinguish between them is by?
- A. passing each gas through bromine water
- B. passing each gas through dilute potassium permanganate solution
- C. passing each gas through silver nitrate solution
- D. treating each gas catalytically with excess hydrogen gas
Three solutions contain carbonate, sulphate and sulphide ions respectively. One test that will identify just ONE of them completely is by addition to each of them of
- A. barium chloride solution
- B. dilute hydrochloric acide
- C. lead nitrate solution
- D. calcium chloride solution
- E. sodium hydroxide solution
If excess zinc is added to a bluish green solution of copper (II) sulphate, and the excess zinc filtered off after completion of reaction, a colourless solution is obtained because
- A. both zinc and copper are metals
- B. the sulphate radical and the zinc ion are divalent
- C. zinc is more electropositive than copper
- D. both zinc and copper form depositive ions in solution
- E. zinc is a reducing agent
Consider the following exothermic reaction
2SO2(g) + O2(g) ↔ 2SO3(g)
If the temperature of the reaction is reduced from 800°C to 500°C, an no other change takes place, then
- A. the reaction rate increases
- B. concentration of SO3 decreases
- C. concentration of SO3 increases
- D. SO2 gas becomes unreactive
- E. O3 gas becomes unreactive
The most common type of chemical reaction which alkanes undergo is
- A. substitution
- B. addition
- C. condensation
- D. polymerisation
- E. double decomposition
Which of the following compounds will form a solution if exposed to air?
- A. Na2CO3.10H2O
- B. NaNO3
- C. CuSO4
- D. CaCl2
- E. Na2SO4.10H2O
The normal boiling point of a liquid is defined as?
- A. the temperature at which its vapour pressure equals the atmospheric pressure
- B. the temperature at which bubbles begin to form
- C. the temperature at which the vapour pressure equals 1 temperature
- D. the temperature at which the rate of condensation of vapour equals the rate of vaporisation of the liquid
- E. the temperature at which the space above the liquid is saturated


