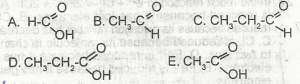
Which of the structural formula above is ethanoic acid is
- A. A
- B. B
- C. C
- D. D
- E. E
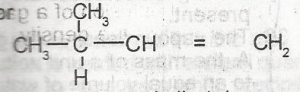
The IUPAC name for
- A. 2-methylbut-3-ene
- B. 2-methylbut-4-ene
- C. 3-methylbut-2-ene
- D. 3-methylbut-1-ene
- E. 3-methylpent-1-ene
Consider the following exothermic reaction \(2SO_{2(g)} + O_{2(g)} = 2SO_{3(g)}\) . If the temperature of the reaction is reduced from 800ºC to 500ºC, and no other change takes place, then
- A. the reaction rate increases
- B. concentration of SO3 decreases
- C. concentration of SO3 increases
- D. SO2 gas becomes unreactive
- E. O2 gas becomes unreactive
Pick out the correct statement
- A. Zin heated to redness reacts with steam to give oxygen and zinc oxide
- B. Zinc heated to redness reacts with steam to give oxygen and hydrogen
- C. Zinc does not react with hot or cold water
- D. Zinc reacts with hot water to form zinc oxide and hydrogen
- E. Zinc reacts very easily with cold water to give zinc oxide and hydrogen
A piece of metal (M) is dissolved in nitric acid and the resulting solution is treated with a small quantity of sodium hydroxide to produce a white precipitate (B) which redissolves on the addition of excess alkali. The precipitate (B)when ignited in a crucible produces the oxide of the metal(m). The METAL M is
- A. Zn
- B. Cu
- C. Al
- D. Au
- E. Ca
Which of the following raw materials would be required from the smelting of iron ore in a blast furnace?
- A. CaCO 3
- B. Zn(NO3)2
- C. CuSO4
- D. AICI3
- E. CaSO4
When excess ethanol is heated to 145 oC in the presence of concentrated sulphuric acid, the product is
- A. diethyl ether
- B. ethyne
- C. diethyl sulphate
- D. acetone
- E. ethanoic acid
A gas occupies 30.0 dm3 at S.T.P. What volume would it occupy at 91oC and 380 mm Hg?
- A. 20. 0dm3
- B. 40.0dm3
- C. 60. 0dm3
- D. 80. 0dm3
- E. 100. 0dm3
One of the following techniques can be used to show that chlorophyll pigment is a mixture of chemical compounds and not a single coloured compound
- A. cystallization
- B. hydrolysis
- C. sublimation
- D. filtration
- E. chromatography
On heating anhydrous iron (ll) sulphate, in addition to producing iron (iii) oxide, which of the following is produced?
- A. SO2 (g) + FeS (s)
- B. SO3 (g) +FeS(s)
- C. SO2 (g) + SO3(g)
- D. SO2 (g) + H2S (g)
- E. SO3(g) + H2S(g)
Phosphorus burns in oxygen according to the equation P4 + 502 → P4O10. How many litres of oxygen will be required at S.T.P for complete oxidation of 12.4g of phosphorus? [P = 31,O = 16 and molar volume of a gas at S.T.P = 22.4 litres]
- A. 5. 20
- B. 11. 20
- C. 2. 24
- D. 20. 20
- E. 6. 20
Bronze is an alloy of
- A. copper, zinc and nickel
- B. aluminium and copper
- C. copper and zinc
- D. tin and zinc
- E. tin and copper
Two metallic ions associated with hard water are
- A. copper and zinc
- B. magnesium and silver
- C. calcium and magnesium
- D. potassium and tin
- E. sodium and lead
Hydrogenation may be effected by
- A. the removal of hydrogen from an alkane in the presence of a catalyst
- B. the addition of hydrogen to an alkane in the presence of a catalyst
- C. the addition of hydrogen to an alkyne
- D. the removal of hydrogen from an alkyne
- E. the addition of hydrogen to an ethyl alcohol
The solution of a sample in a tube will be identified as a chloride if it gives
- A. a white precipitate on addition of AgNO3 and barium chloride solutions
- B. a white precipiate when acidified with HCI, and then AgNO3 solution added
- C. a white precipitate when acidified with dilute HNO3 and then AgNO3 solution added
- D. a white precipitate when acidified with dilute H2SO4 and then barium nitrate solution added
- E. a pungent smell of chlorine gas when dilute HCI and MnO2 are added
The reaction 3C(s) + 2Fe2O3(s) = 4Fe(s) + 3CO2(g): \(\Delta\)H =46.0 kJ is
- A. isothermic
- B. adiabatic
- C. isobaric
- D. endothermic
- E. exothermic
the vapour density of a gas may be defined as
- A. the mass of a unit volume of the gas compared to an equal volume of water vapour
- B. the mass of a unit volume of the gas compared to an equal volume of hydrogen
- C. the mass of a unit volume of the gas compared to an equal volume of oxygen
- D. the mass of a unit volume of the gas minus the vapour pressure of water
- E. two times the relative molecular mass of the gas
Which of the following statements is true?
- A. an increase in the temperature of a given mass of gas increases the number of gas molecules
- B. an increase in the temperature of the gas does not affect the kinetic energy
- C. an increase in the pressure of the gas is proportional to the increase in volume
- D. a increase in the pressure of a gas is proportional to the decrease in volume at constant temperature
- E. a decrease in the pressure of the gas decreases the number of gas molecules present
In the manufacture of iron in the blast furnace, iron (lll) oxide is mixed with coke and limestone, and different reactions occur in the process. Which of the following statements is true with respect to these reactions?
- A. the coke is a powerful reducing agent and easily converts the iron oxide to iron
- B. the calcuim carbonate reacts with SiO2 an earthly impurity in the ore, to from calcuim silicate
- C. the coke will react with the iron produced to form steel
- D. all the carbondioxide required in the process comes from the decomposition of the calcium carbonate
- E. the calcium carbonate decomposes to give calcium oxide, which then forms calcium silicate with the earthly impurity
Weighed salts Y and Z were left exposed in the laboratory overnight. In the morning Y had gained weight, and Z had become liquid. What conclusion could be drawn about the nature of the two salts?
- A. Z is efflorescent
- B. Y and Z are efflorescent
- C. Y and Z are deliquescent
- D. Y and Z are hygroscopic
- E. Y is deliquescent
The reaction between hydrogen and iodine may be represented by the equation H2(g)+ I2(g) → 2HI(g), and is exothermic. Therefore
- A. an increase in temperature favours the forword reaction
- B. an increase in pressure favours the backward reaction
- C. both pressure and temperature muct be increased to favour the forward reaction
- D. a decrease in temperature will favour the forward reaction
- E. a decrease in pressure and an increase in temperature will favour the forward reaction


