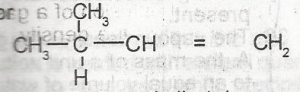
The IUPAC name for
The correct answer is: D
Explanation
Counting the number of Carbon atoms from either way, preference must be given to the Carbon with the double bond. From L-R, the methyl group is on C-2 while counting from R-L, it's on C-3, in terms of the smaller number, it should have been 2-methyl...but since the double bond is on C-1 regardless of the position of the substituent, we'll number in reference to the double bond. Hence, the longest Carbon chain is 4, which is but-1-ene. When the substituent group (Methyl) is added to get the full numenclature which is 3-methyl but-1-ene.


