An endothermic reaction is one during which heat is…..and can be represented by the symbol…. which of the following combinations can be used accurately to complete the above definition?
- A. liberated, -\(\Delta\)H
- B. liberated, +\(\Delta\)H
- C. absorbed, -\(\Delta\)H
- D. absorbed, +\(\Delta\)H
- E. absorbed, +\(\Delta\)G
Which of the following classes of organic compounds undergoes rapid oxidation with bromine water?
- A. alcohols
- B. alkenes
- C. carboxylic acids
- D. esters
- E. alkanes
Detergents are more advantageous than soaps in hard water districts because
- A. on treatment with hard water they either form soluble calcium salts or do not react with calcium ion
- B. on treatment with hard water they form soluble calcium salts
- C. on treatment with hard water they form sodium salts
- D. on treatment with hard water they are converted into soaps
- E. on treatment with hard water they form more lather than soaps does
Which of the following derivatives of nitrogen affords an aqueous solution having a pH of less that 7?
- A. N2O
- B. NO
- C. NO2
- D. NaNO3
- E. NH3
When copper (ii) sulphate solution is treated with excess ammonium hydroxide, a precipitate is first formed, and then this dissolves to give a deep blue solution.
This deep blue solution is a?
- A. Cu(OH)2
- B. CuSo4.5H2O
- C. (NH4)2SO4
- D. Cu(NH3)4(OH)2
- E. CuSO4.(NH4)2SO4.2H2O
Which of the following tests would confirm-unequivocally that a solution X contain the sulphate ion if it gives with?
- A. silver nitrates solution, a white precipates insoluble in dilute HNO3
- B. barium chloride solution, a white precipitate insoluble in dilute H2SO4
- C. barium chloride solution, a white precipitate soluble in dilute HCl
- D. barium chloride solution a white precipitate soluble in dilute ammonia
- E. barium chloride solution a white precipitate insoluble in dilute HCl
The following arrangement of elements in order of decreasing number of outer electrons; One of the elements is wrongly placed. Which is it? ALUMINIUM, CHLORINE, SILICON, MAGNESIUM AND SODIUM.
- A. chlorine
- B. aluminium
- C. silicon
- D. magnesium
- E. sodium
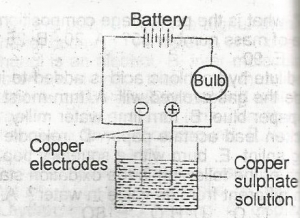
Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed?
- A. The bulb lights and copper is deposited at both electrodes
- B. the bulb lights and copper is deposited at the anode and disappears from the cathode
- C. the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode
- D. the bulb lights and copper is deposited at the cathode and disappears from the anode
- E. the bulb lights but no additional changes are observed
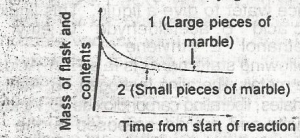
The following graph demonstrates the rate of reaction between calcium carbonate (marble)and dilute hydrochloric acid. The graph shows that the rate of reaction is initially greatest when
- A. the initial slope of the curve is steepest and this occur when small pieces of marble are used and is due to a small surface area of reactant
- B. the occur when large pieces of marble are used and is due to a small surface area of reactant
- C. the initial slope of the curve is steepest and this occurs when small pieces of marble are used and is due to a large surface area of reactant
- D. the initial slope of the curve is steepest and this occur when large surface area of reactant
- E. the initial slope of the curve is least steep and this occur when small pieces of marble are used and is due to the increased surface area of the reactant

Which of the following is the correct order in the above diagram increasing boiling point, of the isomeric \(C_{5}H_{12}\) compounds?
- A. A
- B. B
- C. C
- D. D
- E. E
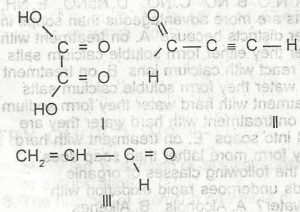
Which of the following compounds will take up the molecules of bromine?
- A. l
- B. ll
- C. lll
- D. l and ll
- E. l and lll
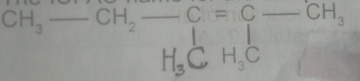
The IUPAC name for the compound is
- A. 2,3-dimethylpent-2,3-ene
- B. 2, 3- dimethylpent-2-ene
- C. 3, 4-dimethylpent-3-ene
- D. 3,4-dimethylpentene
- E. 3,4-dimethlhept-2-ene
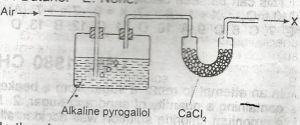
In the above experiment X is?
- A. pure nitrogen
- B. a mixture of nitrogen and oxygen
- C. a mixture of nitrogen and carbondioxide
- D. a mixture of oxygen and inert gases
- E. a mixture of nitrogen and inert gases
When chlorine gas is passed into a solution of sulphur dioxide?
- A. sulphur dioxide is reduced to sulphur
- B. the chlorine is oxidized to hydrochloric acid
- C. dilute sulphuric acid and hydrochloric acid are produced
- D. Hydrochloric acid and sulphur are produced
- E. sulphur trioxide is the only product
A mixture of ethanol, sodium dichromate, and water is allowed to drop gradually into a boiling solution containing one part concentrated H2SO
4 and the liberate gas is collected by cooling it with ice water to give a liquid. This liquid is?
- A. ethanoic acid
- B. acetaldehyde
- C. ethyl acetate
- D. methanol
- E. ethylene
Which of the following statement is false?
- A. Acids react with some metals, liberating hydrogen
- B. Acids react with carbonates, liberating carbondioxide
- C. Acids conduct electricity and are decomposed by the current, liberating hydrogen at the anode
- D. Acids have a sour taste
- E. Acids change the colour of indicators
If one of the following oxides is heated with hydrogen or carbon using a bunsen burner, it is not reduced to the metal. Which one is it?
- A. Lead oxide
- B. Magnesium oxide
- C. Copper oxide
- D. Tin oxide
- E. Iron oxide
Cellulose and starches can be classified as one of the following?
- A. sugars
- B. sucrose
- C. hydrocarbons
- D. carbohydrates
- E. isomers
The best way of collecting chlorine is?
- A. by downward displacement of air
- B. by upward displacement of air
- C. over water
- D. under water
- E. over mercury
A cold aqueous solution of sodium chloride is electrolyzed using a graphite anode and an iron cathode. In the course of the electrolysis the electrolyte is continuously stirred. The liquid left after electrolysis will therefore consist of?
- A. sodium hydroxide solution
- B. chlorine water
- C. pure water
- D. sodium hypochlorite solution
- E. hydrochloric acid solution
Hardening of an oil may achieved by?
- A. boiling the oil with water
- B. boiling the oil with a catalyst
- C. treating the oil with sodium hydroxide
- D. treating the oil with hydrogen and catalyst
- E. distilling oil under reduced pressure


