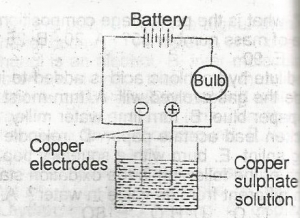
Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed?
The correct answer is: D
Explanation
Copper electrodes are chemically related to CuSO4
Anode: \(Cu^{2+}\) → Cu + 2e
Cathode: \(Cu^{2+}\) + 2e- → Cu
The anode gets thinner, the cathode gets thicker, and work is done in the external circuit to light the bulb.


