Which of the following represents a precipitation reaction?
- A. H2SO4 + NaCl \(\to\) NaHSO4 + HCl
- B. Fe +H2SO4 \(\to\) FeSO4 + H2
- C. 4HCl + MnO2 \(\to\) MnCl2 + 2H2O + Cl2
- D. Na2SO4 + pb(NO3) \(\to\) PbSO4 + 2NaNO
- E. CuO +2HCl \(\to\) Cl2 + H2O
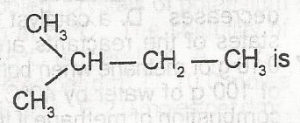
The IUPAC name for
- A. 1-methlpentane
- B. 3-methylbutane
- C. 2-methylbutane
- D. 1-dimethylpropane
- E. 2-methylpentane
The general formula of an alkyl halide (which represent the halide) is?
- A. CnH2n - 2X
- B. CnH2n + 1X
- C. CnH2n + 2X
- D. CnH2nX
- E. CnH2n - 1
An efflorescent compound is a substance that?
- A. absorbs water froom the air without dissolving in it
- B. is capable of giving off coloured luminosity
- C. gives out water to the atmosphere
- D. absorbs water from the air and dissolves in it
- E. gives out its water of crystallization on heating
The scale formation in a kinetic used for boiling water is caused by the presence in water of?
- A. calcium sulphate
- B. calcium carbonate
- C. calcium hydrogen carbonate
- D. calcium hydroxide
- E. magnesium sulphate
What happens when the nitrates of potassium, calcium, zinc and copper are separately heated?
- A. All the nitrate will decompose to their respective metals
- B. The nitrates of calcium and potassium will decomposes to their nitrites
- C. Only copper nitrate decomposes to the metal
- D. Only the nitrates of zinc and copper will decompose to their oxides
- E. The nitrates of calcium, zinc and copper decompose to their oxides
4g each of aluminium foil and aluminium powder were introduced respectively into flasks P and Q.
Flask P was treated with warm bench sodium hydroxide and all the aluminium dissolved in one hour. Flask Q was treated with cold sodium hydroxide and all the aluminium dissolved in 10 minutes. What was responsible for Q reacting faster than P?
- A. There were more aluminium atoms in Q than P
- B. The heat given off in retarded the reaction
- C. Q generated more pressure than P during the reaction
- D. P generated more pressure than Q
- E. Q has a greater surface area for the reaction than P
If one mole of aluminium contains 6 x 1023 atoms of aluminium, how many atoms are contained in 0.9g of aluminium?
(Al = 27)
- A. 1.0 x 1021
- B. 6.6 x 1021
- C. 2.0 x 1022
- D. 1.0 x 1022
- E. 5.4 x 1023
Which one of the following metals is not normally extracted by chemical reduction because of its position in the electrochemical series?
- A. Copper
- B. Iron
- C. Lead
- D. Potassium
- E. Zinc
When hydrogen sulphide and sulphur dioxide are each passed in turn into a solution of potassium dichromate?
- A. both gases change the colour of the solution from yellow to colourless
- B. both gases causes sulphur to be deposited
- C. only hydrogen sulphide decolourizes the solution
- D. only sulphur dioxide deposits sulphur
- E. only hydrogen sulphide deposits sulphur
Which of the following compounds and elements would sublime on exposure to the atmosphere?
- A. Ice
- B. Sulphur
- C. Phosphurous
- D. Iodine
- E. Potassium iodie
What mass of sodium carbonate solution is in 500 cm3 of 0.1 molar sodium carbonate solution?
(Na = 23, C = 12, O = 16)
- A. 10.6 g
- B. 5.3 g
- C. 500 g
- D. 10 g
- E. 20 g
Pure sulfuric acid is a liquid of 1.84 density. What volume of it would be required to prepare 250 cm3 of 2.0 M solution?
(H = 1, S = 32, O = 16)
- A. 2.00
- B. 2.66
- C. 3.00
- D. 3.66
- E. 4.00
In the extraction of iron, which of the following reactions does NOT take place in the blast furnace?
- A. CO2 + C → 2CO
- B. 2Fe2O3 + 3C → 4Fe + 3CO2
- C. Fe2O3 + 3C → 2Fe + 3CO2
- D. CaCO3 → CaO + CO2
- E. CaO + SiO2 → CaSiO3
5cm3 of a saturated solution of sodium chloride at 30°C gave, on careful evaporation, 1.95g of solid salt. The solubility of sodium chloride at 30°C?
(Na = 23, Cl = 35.5)
- A. 5.00 mole dm3
- B. 5.67 mole dm3
- C. 6.00 mole dm3
- D. 6.67 mole dm3
- E. 7.00 mole dm3
The electronic configuration of elements X and Y are X = 2, 8, 6; Y = 2, 8, 7. The bond in the compound formed by X and Y is expected to be?
- A. ionic
- B. covalent
- C. dative covalent
- D. metallic
- E. none of the above
Which of the following is NOT a true statement of the kinetic Theory of Gases?
- A. The molecules move at random
- B. The size of molecules is negligble as compared with the volume of gas
- C. The molecular collisions are perfectly elastic
- D. Every molecule has the same kinetic energy at a particular temperature
- E. The average kinetic energy is proportional to the absolute temperature of the gas
The products of the reaction between dilute sulphuric acid and sodium thiosulphate crystals are?
- A. Na2SO4 + SO2 + O2 + H2O
- B. Na2SO3 + SO3 + O2 + H2O
- C. Na2SO4 + SO2 + S + H2O
- D. Na2SO4 + SO3 + S + H2O
- E. Na2SO4 + SO2 + H2O only
The following compounds are given with their corresponding boiling points.
Compound Boiling Point
i……………………………14°C
ii…………………………..24°C
iii…………………………..0°C
iv…………………………..120°C
V……………………………-2°C
From the above information, indicate which of the following is correct?
- A. All the compounds are gaseous at 20 °C
- B. One of the compounds is gaseous at 20 °C
- C. Two of the compounds are gaseous at 20 °C
- D. Three of the compounds are gaseous at 20 °C
- E. None of the compounds is gaseous at 20 °C
If 48,000 coulombs of electricity are required to discharge 0.5 mole of sodium from a molten sodium chloride mass, then the number of coulombs required to discharge 2 moles of lead from molten lead (ll) chloride is?
- A. 48,000
- B. 96,000
- C. 192,000
- D. 384,000
- E. 576,000
28.8 cm3 of nitrogen at 15°C is cooled to O°C at constant pressure, the new volume of nitrogen is?
- A. 17.4 cm3
- B. 14.7 cm3
- C. 27.3 cm3
- D. 31.7 cm3


