1.00g of mixture of calcium carbonate and calcium oxide liberated 0.33g of carbon dioxide on strong heating. The percentage of calcium oxide in the mixture is? (Ca = 40, C = 12, O = 16)
- A. 5
- B. 15
- C. 25
- D. 35
- E. 50
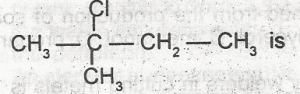
The i.u.p.A.C name for the compound
- A. 2-chloro-isopentane
- B. 3-chloro-isopentane
- C. 2-chloro-2-methylbutane
- D. 1-chloro-2,2- dimethylpropane
- E. 2-chloro-2-ethylpropane
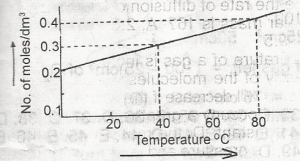
The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be
- A. 10 g
- B. 12 g
- C. 14 g
- D. 16 g
- E. 18 g
60cm3 of hydrogen are sparked with 20cm3 of oxygen at 100°C and 1 atmosphere. The total volume of the residual gases is?
- A. 60 cm3
- B. 10 cm3
- C. 40 cm3
- D. 30 cm3
- E. 70 cm3
The number of grammes of potassium hydroxide in 250cm3 of one molar solution is?
(K = 39, O = 16, H = 1)
- A. 40g
- B. 52g
- C. 100g
- D. 26g
- E. 14g
Brass is an alloy containing copper and?
- A. zinc
- B. tin
- C. aluminium
- D. silver
- E. lead
Which of the following processes NOT lead to a chemical change?
- A. Strirring iron in sulphur (IV) acid
- B. Stirring sodium carbonate in water
- C. Stirring glucose in concentrated sulphur (IV) acid
- D. Mixing sulphur (IV) acid with potassium carbonate
- E. Titrating an acid against base
0.16g of methane when burnt raises the temperature of 100g of water by 40°C. What is the of combustion of methane when if the heat capacity of water is 4.2 Jg-1°C-1?
(CH4 = 16)
- A. 1,160 kJ mol-1
- B. 1,180 kJ mol-1
- C. 1,560 kJ mol-1
- D. 1,600kJ mol-1
- E. 1,680 kJ mol-1
Which of the following is NOT a redox reaction?
- A. 2HNO2 + 2HI → 2H2O + 2NO + I2
- B. Zn + H2SO4 → ZnSO4 + H2
- C. Ca(HCO3)2 → CaCO3 + H2O + CO2
- D. 4FeO + O2 → 2Fe2O3
- E. 2Na + Cl2 → 2NaCl
Which of the following combinations of conditions may increase the rate of a chemical reaction?
- A. Decrease in temperature, increase in concentration of the reactants
- B. Increase in temperature, addition of a catalyst, decrease in the surface area of the reactants
- C. Increase in temperature, increase in concentration, addition of a catalyst and increase in the surface area of the reactants
- D. Decrease in temperature concentration and surface area of the reactants
- E. Additionof a catalyst and in the absence of light
What weight of sodium hydroxide is required to make 500 cm3 of 0.2 M solution?
(Na = 23, O = 16, H = 1)
- A. 40 g
- B. 20 g
- C. 10 g
- D. 4 g
- E. 2 g
How many grams of methylacetylene (propyne) CH3 — C Ξ CH will completely discharge the colour of 8g of bromine?
(Br = 80, C = 12, H = 1)
- A. 0.5
- B. 1.0
- C. 2.0
- D. 3.0
- E. 4.0
Magnesium ribbon was added to each of the following aqueous solution in turn: H2SO4, AgNO3, HNO3, HCl, NH3, HCl, NH3. In which case does the magnesium become covered with a dark precipitate?
- A. H2SO4
- B. AgNO3
- C. HNO3
- D. NH3
- E. HCl
When copper powder is heated with concentrated sulphuric acid, the gas produced is?
- A. hydrogen sulphide
- B. sulphur dioxide
- C. sulphur trioxide
- D. hydrogen pentasulphide
- E. sulphur pentoxide
In the preparation of carbon monoxide by heating ethanedioic acid with concentrated sulphuric acid, the concentrated sulphuric acid act as?
- A. an oxidizing agent
- B. a reducing agent
- C. a dehydrating agent
- D. a reaction medium
- E. a catalyst
A current of 10.0A was passed for 1 hour through a solution of copper (ll) sulphate between the copper electrodes. Which of the following observation would be correct?
(1 Faraday = 96,500 coulombs) (i) 0.186 mole of copper is dissolved from the anode. (ii) 0.372 mole of copper is deposited on the cathode. (iii) the original Cu2+ ion concentration of the solution remains unchanged. (iv) the original Cu2+ ion concentration of the solution decreases.
- A. i and ii
- B. i and iii
- C. i and iv
- D. ii and iii
- E. ii and iv
Which of the following is a neutralization reaction? Addition of
- A. nitric acid to hydrochloric acid
- B. nitric acid to sulphuric acid
- C. nitric acid to distilled water
- D. nitric acid to sodium hydroxide
- E. sodium chloride to distilled water
An element X has two isotopes, \(^{20} _{10}X\) and \(^{22} _{10}X\),
present in the ratio 1:3. The relative atomic mass of X would be?
- A. 20.5
- B. 21.0
- C. 21.5
- D. 22.0
- E. 22.5
When a pressure cooker is half filled with water, and heated to boiling point, then the pressure inside the cooker will?
- A. decrease, since only a fraction of the water molecules has changed to vapour
- B. remain constant, because the total number of water molecules has not changed
- C. increase, because the water molecules can now reach every part of the sealed tube
- D. decrease, since water boils under reduced pressure
- E. increase, because the water vapour molecules now strike the walls of the tube more frequently because of their increased velocity
Which of the following bonds exist in crystalline ammonium chloride (NH4Cl)?
- A. Ionic and covalent
- B. Ionic and co-ordinate
- C. Ionic covalent and co-ordinate
- D. Covalent , co-ordinate and metalllic
- E. Ionic, covalent and metallic
The pressure on 100 cm3 of oxygen gas at 35°C is 750mm of Hg.What would be the volume of the gas if the pressure is increased to 1000mm of Hg without changing the temperature?
- A. 133.3 cm3
- B. 85 cm3
- C. 75 cm3
- D. 65 cm3
- E. 58.3 cm3


