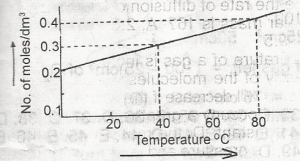
The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be
The correct answer is: D
Explanation
At 80°C, we have \(1000cm^{3} = 0.4mol/cm^{3}\)
40°C = \(0.3mol/cm^{3}\)
hence, the loss in concentration = \(0.1mol/cm^{3}\)
= \(0.1 \times 160 = 16g\)


