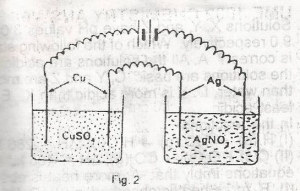
In the experiment above, a current was passed for 10 minutes, and 0.63g of copper was found to be deposited on the cathode of CuSO4 cells. The weight of silver deposited on the cathode of AgNO3 cell during the same period would be? [Cu = 63, Ag = 108]
- A. 0.54g
- B. 1.08g
- C. 1.62g
- D. 2.16g
- E. 3.24g
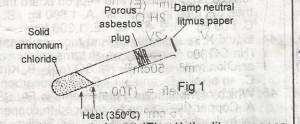
In the above experiment, the litmus paper will initially
- A. be bleached
- B. turn green
- C. turn red
- D. turn blue
- E. turn black
30 cm3 of 0.1 M Al (NO3)3 SOLUTION IS RECTED WITH 100cm3 of 0.15M of NaOH solution. Which reactant is in excess, and by now much ?
- A. NaOH solution by 70 cm3
- B. NaOH solution, by 60 cm3
- C. NaOH solution by 40 cm3
- D. Al(NO3)3 solution by 20 cm3
- E. Al(NO3)3 solution by 10 cm3
\(Zn + H_2 SO_4 → ZnSO_4 + H_2\)
in the above reaction, how much zinc will be left undissolved if 2.00g of zinc is treated with 10cm^3 of 1.0 M of \(H_2SO_4\)?
[Zn =65, s = 32, O = 16, H = 1]
- A. 1.35g
- B. 1.00g
- C. 0.70g
- D. 0.65g
- E. 0.00g
zn + 2HCl → ZnCl2 + H2
The rate of the above reaction will be greatly increased if
- A. the zinc is in the powered form
- B. a greater volume of the acid is used
- C. a smaller volume of the acid is used
- D. the reaction vessel is immersed in an ice-bath
- E. the zinc is in the form of pellets
The equilibrium reaction between copper (l) chloride and chlorine at 25oC and 1 atmosphere is represented by the equation:
2CuCl +Cl2 ⇌ 2 CuCl2 ∆H = – 166 KJ. Which of the following statements is TRUE for the reaction, pressure remaining constant?
- A. more CuCl is formed at 40oC
- B. more CuCl2 is formed at 10oC
- C. Less CuCl2 is formed at 10oC
- D. there is no change in the amount of CuCl2 formed at 40oC and10oC
- E. more CuCl is consumed at 40oC
When sodium dioxonitrate (lll) (NaNO2) dissolves in water, ∆H is positive. The process of dissolution is
- A. exothermic
- B. endothermic
- C. isothermal
- D. isomeric
- E. hygroscopic
Which of the following statements is FALSE?
- A. Copper (ll) ion can be reduced to copper (l) ion by hydrochloric acid and zinc
- B. Sodium metal dissolves in water giving oxygen
- C. Nitrogen is insoluble in water
- D. Carbondioxide is soluble in water
- E. lead has a higher atomic weight than copper
Liquid X, reacts with sodium trioxocarbonate (IV) (Na2CO3) to give a gas which turns calcium chloride solution milky X is
- A. Na2SO4(aq)
- B. Kl (aq)
- C. an alkali
- D. an acid
- E. a hydrocarbon
A jet plane carrying 3,000 kg of ethane burns off all the gas forming water and carbondioxide. if all the carbondioxide is expelled and all the water formed is condensed and kept on board the plane, then the gain in weight is
- A. 1,800 kg
- B. 900 kg
- C. 600 kg
- D. 2, 400 kg
- E. 1, 200 kg
Which of the following is a neutralization reaction?
- A. Addition of sodium chloride solution to potassium chloride solution
- B. Addition of trioxonitrate (V) acid (nitric acid) to distilled water
- C. Addition of trioxonitrate (V) acid (nitric acid) to tetraoxosulphate (IV) acid (sulphuric acid)
- D. Addition of trioxonitrate (V) acid (nitric acid) to potassium trioxonitrate (V) (potassium nitrate) solution
- E. Addition of trioxonitrate (V) acid (nitric acid) to potassium hydroxide solution
The mass of an atom is determined by
- A. its ionization potential
- B. its electrochemical potential
- C. the number of protons and electrons
- D. the number of neutrons and protons
- E. the number of neutrons and electrons
Which of the following solutions will give a white precipitate with barium chloride solution and a green flame test?
- A. Na2SO4
- B. CuSO4
- C. CaSO4
- D. CaCI2
- E. (NH4)2SO4
A gas that can behave as a reducing agent towards chlorine and as an oxidizing agent toward hydrogen sulphide is
- A. O2
- B. NO
- C. SO2
- D. NH3
- E. CO2
The cracking process is very important in the petroleum industry because it.
- A. gives purer products
- B. yields more lubricants
- C. yields more engine fuels
- D. yields more asphalt
- E. yields more candle wax
3. 06 g of a sample of potassium trioxochlorate (V) (KCIO\(_3\)) was required to make a saturated solution with 10cm\(^3\) of water at 25°C. The solubility of the salt at 25°C is [K =39, Cl = 35.5, O = 16]
- A. 5.0 moles dm3
- B. 3.0 moles dm3
- C. 2.5 moles dm3
- D. 1.0 molesv
- E. 0.5 moles dm3
2.5 g of a hydrate barium salt gave on heating, 2.13 g of the anhydrous salt. Given that the relative molecular mass of the of the anhydrous salt is 208, the number of molecules of water of crystallization of the barium salt is
- A. 10
- B. 7
- C. 5
- D. 2
- E. 1
Increasing the pressure of a gas
- A. lowers the average kinetic energy of the molecules
- B. decreases the density of the gas
- C. decreases the temperature of the gas
- D. increases the density of the gas
- E. increases the volume of the gas
Which of the following separation processes is most likely to yield high quality ethanol (>95%) from palm wine?
- A. Fractional distillation without a dehydrant
- B. simple distillation with a dehydrant
- C. fractional distillation with a dehydrant
- D. column chromatography
- E. evaporation
In the preparation of some pure crystals of Cu (NO3)2 starting with CuO, a student gave the following statements as steps he employed. Which of these shows a flaw in his report ?
- A. some CuO was reacted with excess dilute H2SO4
- B. the solution was concentrated
- C. when the concentrate was cooled, crystals formed were removed by filtration
- D. the crystals were washed with very little cold water
- E. the crystals were then allowed to dry
Some metals are extracted from their ores after some preliminary treatments by electrolysis (L), some by thermal decomposition(T), some by a combination of both processes (TL). Which set-up in the following for the extraction of iron, copper, and aluminium is correct?
- A. lron (L), copper (L) aluminium (T)
- B. lron (T) copper (L) aluminium (T)
- C. lron (TL) copper (TL) aluminium (TL)
- D. Iron (L) copper (T) aluminium (T)
- E. lron (T) copper (L), aluminium (TL)


