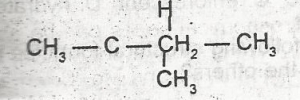
The I.U.P.A.C name for the compound is
- A. isopropylethene
- B. acetylene
- C. 3- methylbutane
- D. 2-methylbutane
- E. 5-methylpentane
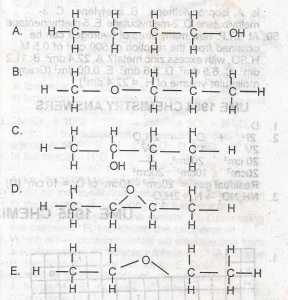
Use the diagram above to answer this question
Which of the following structural formula is NOT isomeric with the others?
- A. A
- B. B
- C. C
- D. D
- E. E
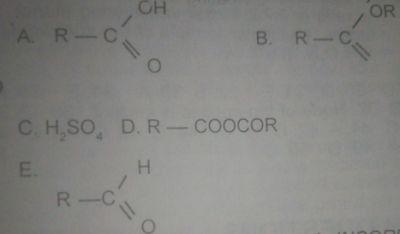
USE THE DIAGRAM ABOVE TO ANSWER THIS QUESTION.
Which of the following represents a carboxylic acid?
- A. A
- B. B
- C. C
- D. D
- E. E
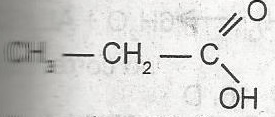
The name of the compound above is
- A. acetic acid
- B. propanal
- C. propanol
- D. ethanoic acid
- E. propanoic acid
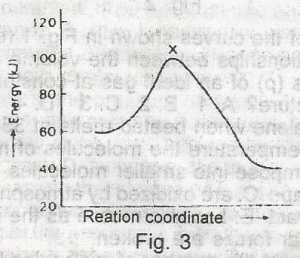
The diagram above (Fig.3) shows the energy profile for the reaction A + B + = C + D From this diagram, it is clear that the reaction is
- A. spontaneous
- B. isothermal
- C. adiabatic
- D. exothermic
- E. endothermic
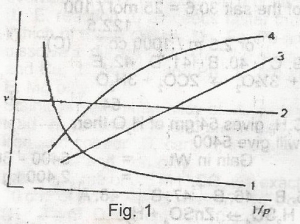
Which of the curves shown in the figure given represents the relationships between the volume(V) and pressure(P) of an ideal gas at constant temperature
- A. 1
- B. 2
- C. 3
- D. 4
- E. 1 and 3
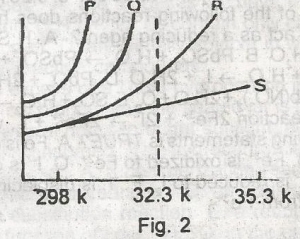
The solubility curves of four substances are shown in the figure above. Which of the four substances would crystallize from a saturated solution cooled from 353K(80oC)to 323K(50C)?
- A. P and Q
- B. P and R
- C. P and S
- D. R and S
- E. Q and R
At S. T. P how many litres of hydrogen can be obtained from the reaction of 500 cm3 of 0.5 M H 2SO 4 with excess zinc metal?
(Gram molecular volume of H2 = 22. 4 dm 3)
- A. 22.4 dm 3
- B. 11 . 2 dm 3
- C. 6. 5 dm 3
- D. 5. 6 dm 3
- E. 0. 00 dm 3
An aqueous solution of a metal salt, M, gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia. Therefore cartion in M is
- A. Zn ++
- B. Ca++
- C. Ai+++
- D. Pb++
- E. Cu++
Which of the following electrodes is used in electrolysis of bauxite (Al2O3) in molten cryolite?
- A. Steel electrodes
- B. Carbon electrodes
- C. Mercury electrodes
- D. Carbon anode and steel cathode
- E. Carbon cathode and stell anode
A piece of sea shell, when dropped into a dilute solution of hydrochloric acid, produces a colourless, odourless gas which turns clear limewater milky. The sea shell contains
- A. sodium chloride
- B. ammonium nitrate
- C. calcium carbonate
- D. calcium chloride
- E. magnesium chloride
The function of conc. H2SO4 in the esterification of ethanoic acid with ethanol is to
- A. serve as a dehydrating agent
- B. serve as a solvent
- C. act as a catalyst
- D. prevent any side reaction
- E. serve as an oxidizing agent
If an excess of a liquid hydrocarbon is poured into a jar of chlorine, and the sealed jar is then exposed for several hours to bring sunlight, all the chlorine gas is consumed. The hydrocarbon is said to have undergone
- A. a polymerization reaction
- B. an isomerization reaction
- C. an addition reaction
- D. a substitution reaction
- E. a reduction reaction
Alkanes
- A. are all gases
- B. have the general formula CnH2n + 2O
- C. contain only carbon and hydrogen
- D. are usually soluble in water
- E. are usually active compounds
Sodium hydroxide (NaOH) pellets are
- A. deliquescent
- B. hygroscopic
- C. efflorescent
- D. hydrated
- E. fluorescent
Water is said to be ‘hard’ if it
- A. easily forms ice
- B. has to be warmed before sodium chloride dissolves in it
- C. froms an insoluble scum with soap
- D. contains nitrates
- E. contains sodium ions
Nitrogen can best be obtained from a mixture over oxygen and nitrogen by passing the mixture over
- A. potassium hydroxide
- B. heated gold
- C. heated magnesium
- D. heated phosphorus
- E. calcium chloride
If the cost of electricity required to deposit 1 g of magnesium is N5.00, how much would it cost to deposit 10g of aluminium?
- A. N 10.00
- B. N27.00
- C. N44.44
- D. N66.67
- E. N33.33
Which of the following statements is NOT correct about all four of the acids: HBr, HNO3, H 2CO3 and H2SO4? They
- A. dissolve marble to liberate CO
- B. have a PH less than 7
- C. Turn blue litmus red
- D. neutralize alkalis to form salt
- E. react with magnesium to liberate hydrogen
Which of the following statements is INCORRECT?
- A. Fractional distillation of crude petroleum will give the folloing hydrocarbon fuels in order of increasing boiling point
- B. Butane < petrol < kerosene of polythene.
- C. Both but-1-ene and but-1-yne will decolorize bromine readily
- D. but-2-ene will react with chlorine to form 2,3-dichlorobutane
- E. calcuim carbide will react with water to form any alkyne
Alkaline hydrolysis of naturally occurring fats and oils yields
- A. fats and acids
- B. soaps and glycerol
- C. margarine and butter
- D. esters
- E. detergents


