Copper sulphate solution is electolysed using platinum electrodes A. current of 0.193 amperes is passed for 2hrs. How many grams of copper are deposited? (cu = 63.5, F = 96500 coulombs)
- A. 0.457g
- B. 0.500g
- C. 0.882g
- D. 0.914g
- E. 1.00g
In what respect will two dry samples of nitrogen gas differ from each other if sample 1 is prepared by completely removing CO2 and O2 from air, and sample 2 is prepared by passing purified nitrogen(i)oxide over heated copper? sample 1 is
- A. purer than sample 2
- B. slightly denser than sample 2
- C. in all respects the same as sample 2
- D. colourless but sample 2 has a light brown colour
- E. slightly less reactive than sample 2
The boiling point of water, ethanol, toluene and butan-2-ol are 373.0k, 351.3k, 383.6k and 372.5k respectively. Which liquid has the highest vapour pressure at 323.0k?
- A. water
- B. toluene
- C. ethanol
- D. butan-2-ol
- E. none
Hydrogen is not liberated when trioxonitrate(v)acid reacts with zinc because
- A. zinx is not rendered passive by acid
- B. hydrogen produced is oxidized to water
- C. oxides of nitrogen are produced
- D. all nitrates are soluble in water
- E. trioxonitrate(v)acid is a strong acid
A certain volume of gas at 298k is heated such that its volume and pressure are now four times the original values. What is the new temperature?
- A. 18.6k
- B. 100.0k
- C. 298.0k
- D. 1192.0k
- E. 4768.0k
Some properties of chemical substances are mentioned below… i. sour taste ii. slippery touch iii. yields alkaline gas with ammonium salt iv. has pH less than 7 v. turns phenolphthalein pink.
Which of the above are NOT typical properties of alkalis?
- A. i, iv and v
- B. iv and v
- C. i and iv
- D. ii and v
- E. ii, iii and iv
3.0g of a mixture of potassium carbonate and potassium chloride were dissolved in a 25cm3 of this solution required required 40.00cm3 of 0.1M HCl for neutralization. What is the percentage by weight of K2CO3 in the mixture. (K = 39, O = 16, C = 12)
- A. 60
- B. 72
- C. 82
- D. 89
- E. 92
Given that the molecular mass of iron is 56 and that of oxygen is 16, how many moles of iron(iii)oxide will be contained in 1kg of the compound?
- A. 25.0 moles
- B. 12.5 moless
- C. 6.25 moles
- D. 3.125 moles
- E. 0.625 moles
Hydrogen diffuses through a porous plug
- A. at the same rate as oxygen
- B. at a slower rate than oxygen
- C. twice as fast as oxygen
- D. three times as fast as oxygen
- E. four times as fast as oxygen
In the experiment, which of the following observations would suggest that a solid sample is a mixture? The
- A. solid can be ground to a fine powder
- B. density of the solid is 2.25gdm-3
- C. solid begins to melt at 573k but is not completely melted until 648k
- D. solid absorbs moisture from the atmosphere and turns into liquid
- E. solid melts at 300k
0.499g 0f CuSO4.xH2O when heated to constant weight gave a residue of 0.346g. the value of x is? (Cu = 63.5, S = 32.0, O = 16, H = 1)
- A. 0.5
- B. 2.0
- C. 3.0
- D. 4.0
- E. 5.0
An organic compound contains 72% carbon, 12% hydrogen and 16% oxygen by mass. The empirical formula of the compound is
- A. C8H22O3
- B. C6H10O3
- C. C12H12O
- D. C6H12O
- E. C3H6O
Which of the following conduct electricity?
- A. sulfur
- B. graphite
- C. diamond
- D. red phosphorus
- E. yellow phosphorus

Use the following option above to answer this question. Which of the following is the functional group of carboxylic acids?
- A. A
- B. B
- C. C
- D. D
- E. E
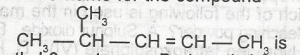
The I.U.P.A.C name for the compound
- A. 2-methyl-3-pentene
- B. 4-methyl-2-pentane
- C. 2-methyl-2-pentene
- D. 4-methylpent-2-ene
- E. 2-methyl-3-pentane
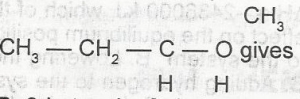
The oxidation of
- A. 2-butanone
- B. 2-butanal
- C. butane
- D. butanoic acid
- E. 3-butanal
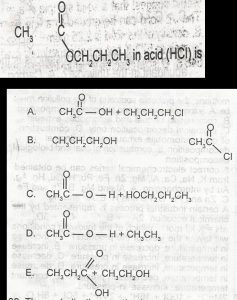
Use the above options to the question. The products formed on hydrolysis of
- A. A
- B. B
- C. C
- D. D
- E. E
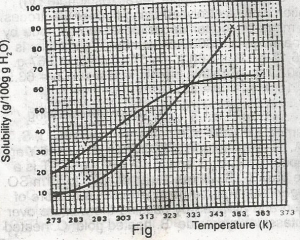
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
- A. 0.2 moles
- B. 0.7 moles
- C. 1.5 moles
- D. 2.0 moles
- E. 3.0 moles

The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If 80 g each of X and Y are taken up in 100 g of water at 353 K we shall have
- A. only 10g of X undissolved
- B. only 16 g of Y undissolved
- C. 10 g of X and 16 g of Y undissolved
- D. all X and Y dissolved
- E. all X and Y undissolved
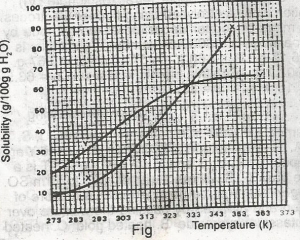
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. At room temperature (300 K)
- A. Y is twice as soluble as X
- B. X is twice as soluble as Y
- C. X and Y are soluble to the same extent
- D. X is three times as soluble as Y
- E. Y is three times as soluble as X
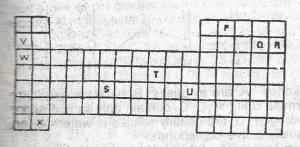
Figure 1 above shows part of the periodic Table which of the elements belongs to the p-block?
- A. S, T and U
- B. V, W and X
- C. S and T only
- D. P, Q and R
- E. V, W, X and S


