When 50cm3 of a saturated solution of sugar (molar mass 342.0g) at 40oC was evaporated to dryness, 34.2g of dry solid was obtained. The solubility of sugar at 40oC is
- A. 10.0 moles dm-3
- B. 7.0 moles dm-3
- C. 3.5 moles dm-3
- D. 2.0 moles dm-3
Which of the following groups of physical properties increases from left to right of the periodic table?
i. Ionization energy ii. Atomic radius iii. Electronegativity iv. Electron affinity
- A. i and ii
- B. i, ii and iii
- C. iii and iv
- D. i, iii and iv
An element with atomic number twelve is likely to be
- A. electrovalent with a velocity of 1
- B. electrovalent with a valency of 2
- C. covalent with a valency of 2
- D. covalent with valency of 4
The relationship between the velocity (U) of gas molecules and their relative molecular mass (M) is shown by the equation
- A. U = (kM)\(\frac{1}{2}\)
- B. U = (kM)2
- C. U = \(\sqrt{\frac{k}{m}}\)
- D. U = \(\frac{k}{m}\)
How much of magnesium is required to react with 260cm3 of 0.5 M HCl? [Mg = 24]
- A. 0.3g
- B. 1.5g
- C. 2.4g
- D. 3.0g
The number of atoms of chlorine present in 5.85g of NaCl is [Avogaro’s Number = 6.02 x 1023]
- A. 6.02 x 1022
- B. 5.85 x 1023
- C. 6.02 x 1023
- D. 5.85 x 1024
10cm3 of hydrogen fluoride gas react with 5cm3 of dinitrogen difluoride gas (N2F2) to form 103 of a single gas. Which of the following is the most likely equation of the reaction?
- A. HF + N2F2 \(\to\) N2HF3
- B. 2HF + N2F2 \(\to\) 2NHF2
- C. 2HF + N2F2 \(\to\) N2H2F3
- D. HF + 2N2F2 \(\to\) N4HF2
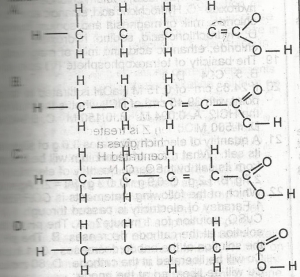
Use the options above to answer this question. Which is the structural formula for pent-2-enoic acid?
- A. A
- B. B
- C. C
- D. D

The tabulated results below were obtained by titration 10.0 cm3 of water with soup. The titration was repeated with the same sample of water after boiling. The ratio of permanent to temporary hardness is
- A. 1.5
- B. 1.4
- C. 4.1
- D. 5.1
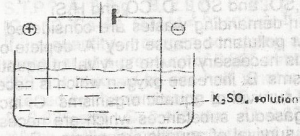
In the electrolysis of aqueous solution of K2SO 4 in the above cell, which species migrate to the anode?
- A. SO2-OH -
- B. K + and SO 4 2-
- C. OH and HO
- D. H3O + and K
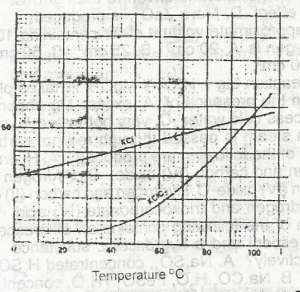
In the solubility curve above, water at 98oC is saturated with KCL and KCIO 3 What is the percentage of KCL impurity in the crystals formed when the solution is cooled to 30 oC?
- A. 51.5
- B. 45.5
- C. 34.5
- D. 26.5
What mass of a divalent metal M (atomic mass = 40) would react with excess hydrochloric acid to liberate 224cm^3 dry hydrogen gas measured at S.T.P?
- A. 8.0 g
- B. 4.0 g
- C. 0.8 g
- D. 0.4 g
The movement of liquid molecules from the surface of the liquid to the gaseous phase above it is known as?
- A. Brownian movement
- B. condensation
- C. evaporation
- D. liquefaction
Starch can be converted to ethyl alcohol by?
- A. distillation
- B. fermentation
- C. isomerization
- D. cracking
Which of the following are made by the process of polymerization?
- A. Nylon and soap
- B. Nylon and artificial rubber
- C. Soap and butane
- D. Margarine and nylon
The general formula of an alkyl halide (where X represents the halide) is?
- A. CnH2n 2X
- B. CnH2n + 1X
- C. CnH2n + 2X
- D. CnH2nX
Which of the following substance is a mixture?
- A. Granulated sugar
- B. Sea-water
- C. Sodium chloride
- D. Iron fillings
Which of the following compounds is NOT formed by the action of chlorine on methane?
- A. CH3CI
- B. C2H5CI
- C. CH2CI2
- D. CHCI3
When ethanol is heated with excess concentrated sulphuric acid, the ethanol is?
- A. oxidized to ethene
- B. polymerized to polyethene
- C. dehydrated to ethene
- D. dehydrated to ethyne
What reaction takes place when palm-oil is added to potash and foam are observed?
- A. Neutralization
- B. Saponification
- C. Esterification
- D. Salting-out
Which of the following is used as an ‘anti-knock’ in automobile engines?
- A. Tetramethyl silane
- B. Lead tetra-ethyl
- C. Glycerol
- D. n-heptane


