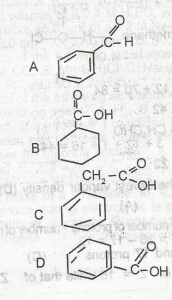
Use the option above to answer this question. The structure of benzoic acid is
- A. A
- B. B
- C. C
- D. D

The times taken for iodine to be liberated in the reaction between sodium thiosulphate and hydrochloric acid at various temperature are as follows. These results suggest that
- A. for a 10o rise in temperature, rate of reaction is double
- B. for a 10 o rise in temperature, rate of reaction is halved
- C. time taken for iodine to appear does not depend on temperature
- D. for a 10 o rise in temperature rate of reaction is tripled

Use the option above to answer this question. Which is the correct set of results for tests conducted respectively on fresh lime juice and ethanol.
- A. A
- B. B
- C. C
- D. D
An element Z, contained 90% of 168Z and 10% of Z1818 its relative atomic mass is?
- A. 16.0
- B. 16.2
- C. 17.0
- D. 17.8
An isotope has an atomic number of 17 and a mass number of 36. Which of the following gives the correct number of neutrons and protons in an atom of the isotope?
- A. Neutrons 53, Protons 17
- B. Neutrons 17, Protons 36
- C. Neutrons 19, Protons 17
- D. Neutrons 36, Protons 17
If d represents the density of a gas and K is a constant, the rate of gaseous diffusion r is related to d by the equation?
- A. r = k/√d
- B. r = kd
- C. r =k/d
- D. r = k √d
The atomic numbers of two elements X and Y are 12 and 19 respectively. The bond of these two elements is?
- A. ionic
- B. covalent
- C. Metallic
- D. co-ordinate
If 24.83 cm3 of 0.15 M NaOH is titrated to its end point with 39.45 cm3
of HCl, what is the molarity of the HCl?
- A. 0.094 M
- B. 0.150 M
- C. 0.940 M
- D. 1.500 M
In which of the following are the aqueous solutions of each of the substances correctly arranged in order to decreasing acidity?
- A. Ethanoic acid, milk or magnesia, sodium chloride, hydrochloric acid and sodium hydroxide
- B. Ethanoic acid, hydrochloric acid, milk of magnesia, sodium chloride and sodium chloride and sodium hydroxide
- C. Hydrochloride acid, ethanol acid, sodium chloride, milk of magnesia and sodium hydroxide
- D. Hydrochloride acid, sodium hydroxide sodium chloride, ethanol acid, and milk of magnesia
The solubility of Na3AsO4(H2O)12 is 38.9g per 100g H2O. What is the mass percentage of Na3AsO4 in the saturated solution?
(As = 75, Na = 23, O = 16, H = 1)
- A. 87.27%
- B. 38.9%
- C. 19.1%
- D. 13.7%
Oil spillage in ponds and creeks can be cleaned up by?
- A. burning off the oil layer
- B. spraying with detergent
- C. dispersal with compressed air
- D. spraying with hot water
Which of the following water samples will have the highest titre value when titrated for the Ca2+ ions using soap solution?
- A. Permanently hard water after boiling
- B. emporarily hard water after boiling
- C. Rain water stored in a glass jar for two years
- D. Permanent hard water passed through permuitit
In the purification of town water supply, alum is used principally to?
- A. kill bacteria
- B. control the pH of water
- C. improve the taste of the water
- D. coagulate small pasrticles of mud
A stream of air was successively passed through three tubes X, Y and Z containing a concentrated aqueous solution of KOH, red hot copper powder and fused calcium chloride respectively. What was the composition of the gas emanating from tube Z?
- A. CO2 ad ithe inert gases
- B. N2, CO2 and the inert gases
- C. N2 and the inert gases
- D. water vapour, N2 and the inert gases
The greater the different in electronegativity between bonded atoms, the?
- A. lower the polarity of the bond
- B. higher the polarity of the bond
- C. weaker the bond
- D. higher the possibility of the substance formed being a molecule
When water drops are added to calcium carbide in a container and the gas is passed through a jet and lifted, the resultant flame is called an?
- A. oxyethylene flame
- B. oxyhydrocarbon flame
- C. oxyacetylene flame
- D. oxymethane flame
Complete oxidation of propan -1- ol gives?
- A. propanal
- B. propan-2-al
- C. propan-1-one
- D. propanoic acid
Synthetic rubber is made by polymerization of?
- A. 2 methyl buta - 1, 3 - diene
- B. 2 methyl buta - 1, 2 - diene
- C. 2 methyl but -1-ene
- D. 2 methyl but-2-ene
Local black soap is made by boiling palm oil with liquid extract of ash. The function of the ash is to provide the?
- A. acid
- B. ester of allanoic acid
- C. alkali
- D. alkanol
The most volatile fraction obtained from fractional distillation of crude petroleum contains?
- A. butane , propane and kerosene
- B. butane , propane and petrol
- C. ethane, methane and benzene
- D. methane, ethane and propane
Ethanol reacts with aqueous sodium mono-oxoio date (l) to give a bright yellow solid with a characteristic smell. The product is?
- A. trichloromethane
- B. triodomethane
- C. iodoethane
- D. ethanal


