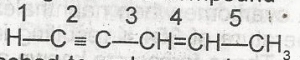
The acidic hydrogen in the compound is the hydrogen attached to carbon number
- A. 5
- B. 4
- C. 3
- D. 1
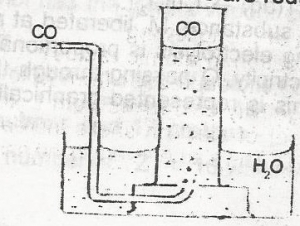
Carbon (ll) oxide may be collected as shown above because
- A. is heavier than air
- B. is less dense than air
- C. is insoluble in water
- D. burns in oxygen to form carbon (IV) oxide
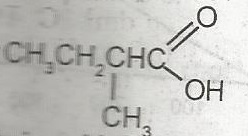
The IUPAC name for
- A. 2-methylbutanoic acid
- B. 2-methyl-1- hydroxyketone
- C. 2-methyl-1-hydroxy aldehyde
- D. 2-methylpentanoic acid
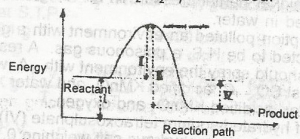
The diagram above shows the reaction path of an exothermic reaction. The heat of reaction is represented by
- A. l
- B. ll
- C. lll
- D. lV
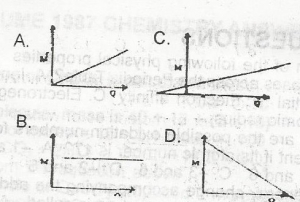
Use the graph above to answer this question. The mass of a substance, M, liberated at an electrode during electrolysis is proportional to the quantity of electricity, Q passing through the electrolyte. This is represented graphically by.
- A. A
- B. B
- C. C
- D. D
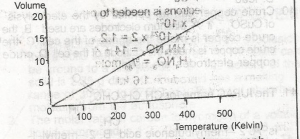
Which of the gas laws does the above graph illustrate?
- A. Boyle
- B. Charles
- C. Graham
- D. Gay-Lussac
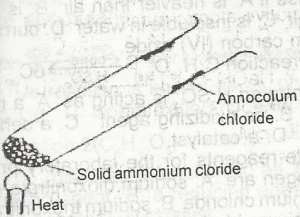
In the experiment above, ammonium chloride crystals deposit on the walls of the tube is as a result of
- A. evaporation
- B. recrystallization
- C. sublimation
- D. fractional precipitation
An eruption pollution an environment with gas suspected to be H2S, a poisonous gas. A residue team should spray the environment with?
- A. water
- B. moist SO2
- C. acidified KMnO4 and water
- D. water, acidified KMnO4 and oxygen
The molar ratio of oxygen to nitrogen in dissolved air is 2:1 whereas the ratio is 4:1 in atmospheric air because?
- A. nitrogen is less soluble than oxygen
- B. oxygen is heavier than nitrogen
- C. nitrogen has a higher partial pressure in air
- D. gases are hydrated in water
The energy changes accompanying the addition of an electron to a gaseous atom is called?
- A. first ionization energy
- B. second ionization energy
- C. electron affinity
- D. electronegativity
What are the possible oxidation numbers of an element if its atomic number is 17?
- A. -1 and 7
- B. -1 and 6
- C. -3 and 5
- D. -2 and 6
Which of the following physical properties decreases across the Periodic Table?
- A. Ionization potenial
- B. Electron affinity
- C. Electronegativity
- D. Atomic radius
A metallic ion X2+ with an inert gas structure contains 18 electrons. How many protons are there in this ion?
- A. 20
- B. 18
- C. 16
- D. 2
The forces holding naphthalene crystals together can be overcome when naphthalene is heated to a temperature of 354K resulting in the crystal to melting. These forces are known as?
- A. coulombic
- B. ionic
- C. covalent
- D. Van der waals
An increase in temperature causes an increase in the pressure of a gas because there is an increase in the?
- A. average velocity of the molecules
- B. number of collisions between the molecules
- C. density of the molecules
- D. free mean path between each molecules and other
One mole of propane is mixed with five moles of oxygen. The mixture is ignited and the propane burns completely. What is the volume of the products at s.t.p?
(G.M.V = 22.4 dm3 mol-1)
- A. 112.0 dm3
- B. 67.2 dm3
- C. 56.0 dm3
- D. 44.8 dm3
2.25g of a sample of an oxide of copper on reduction gave 2.0g of copper. 2.50g of another oxide of copper on reduction also gave 2.0g of copper. These results are in accordance with the law of?
- A. constant composition
- B. conservation of matter
- C. multiple proportions
- D. definite proportions
The formula of a compound formed in a reaction between a trivalent metal M and a tetravalent non-metal X is~?
- A. MX
- B. M3X4
- C. M4X3
- D. M3X2
What process would coal undergo to give coal gas, coal tar, ammoniacal liquor and coke?
- A. Steam distillation
- B. Destructive distillation
- C. Liquefaction
- D. Hydrolysis
Detergents have the general formula?
- A. R(CH2)nOH
- B. RSO3-Na+
- C. RSO-2Na+
- D. RSO2H
Which of the following compounds has the highest boiling point?
- A. CH3CH2CH2CH2HO
- B. CH3CH2CH2CHO
- C. CH3CH2CH2CH3
- D. CH3CH2OCH2CH3


