CO(g) + H2O(g) \(\to\) \(CO_{2g}\) + H2(g) \(\Delta\)H = -4100J. Which of the following factors favour the formation of hydrogen in the above reaction? i. high pressure. ii. low pressure iii. high-temperature iv. use of excess steam
- A. i, iii and iv
- B. iii only
- C. ii, iii and i
- D. iv only
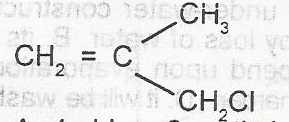
What is the IUPAC name for the compound
- A. 1-chloro-2-methylprop-2,3-ene
- B. 1-chloro-2-methylprop-2-ene
- C. 3-chloro-2-methylprop-1-ene
- D. 3-chloro-2methylprop-1,2-ene
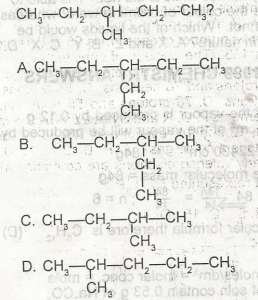
Use the figure above to answer this question. Which of the following is NOT a monomer?
- A. A
- B. B
- C. C
- D. D
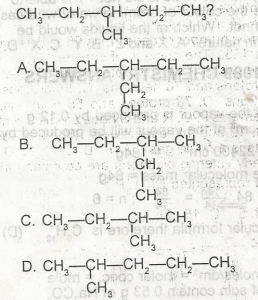
Use the figure above to answer this question. Which of the following compounds is an isomer of the compound
- A. A
- B. B
- C. C
- D. D
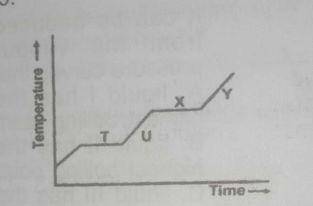
The above graph shows a typical heating curve from the solid phase through the liquid phase to the gaseous phase of a substance. Which part of the curve shows solid and liquid in equilibrium?
- A. T
- B. U
- C. X
- D. Y
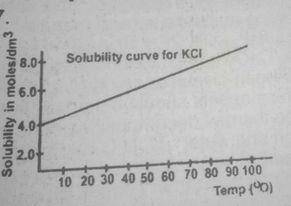
If in the graph above 1 dm 3 of a saturated solution of KCL is cooled from 80°C, the mass of crystals deposited will be
[K = 39, Cl = 35.5]
- A. 7.45 g
- B. 14.90 g
- C. 74.50 g
- D. 149.00 g
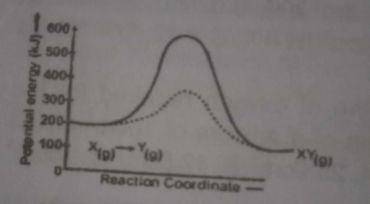
The above diagram gives the potential energy profile of the catalyst and uncatalysed reactions of X(g) + Y(g) → XY(g). Deduce the respective activation energies in KJ of the catalysed and uncatalysed reverse reactions: XY(g) → X(g) + Y(g)
- A. 300, 500
- B. 500, 300
- C. -300, -500
- D. -500, -300
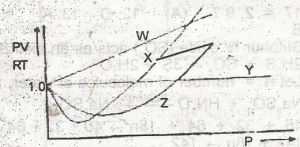
Which of the curves in the above graph illustrates the behaviour of an ideal gas?
- A. W
- B. X
- C. Y
- D. Z

In the above set up substances X and Y are respectively
- A. lime water copper (ll) tetraoxosulphate (lV)
- B. potassium trioxocarbonate and alkaline pyrogallol
- C. potassium hydroxide and alkaline pyrogallol
- D. potassium trioxocarbonate (lV) and concentrated tetraoxosulphate (IV) acid
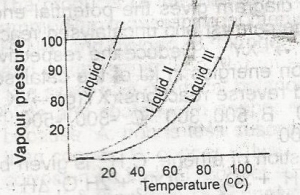
It can be deduced from the vapour pressure curves above that
- A. liqiud 1 has the highest boiling point
- B. liquid ll has the highest boiling point
- C. liquid lll has the highest boiling point
- D. liquid lll has the lowest boiling point
Three liquids X, Y and Z containing only hydrogen and carbon were burnt on a spoon. X and Y burnt with sooty flames while Z did not. Y is able to discharge the colour of bromine water whereas X and Z cannot. Which of the liquids would be aromatic in nature?
- A. X and Z
- B. Y
- C. X
- D. Z
The gas responsible for most of the fatal explosions in coal mines is
- A. butane
- B. ethene
- C. ethane
- D. methane
The alkaline hydrolysis of fats and oils produces soap and
- A. propane 1, 1, 3 - triol
- B. propane - 1,2,3 - triol
- C. propane - 1,3,3 - triol
- D. propane - 1, 2, 2 - triol
The bond which joins two ethanoic acid molecules in the liquid state is
- A. a covalent bond
- B. an ionic bond
- C. a dative covalent bond
- D. a hydrogen bond
The reaction between ethanoic acid and sodium hydroxide is an example of
- A. esterification
- B. neutralization
- C. hydroxylation
- D. hydrolysis
Vulcanization of rubber is a process by which
- A. isoprene units are joined to produce rubber
- B. rubber latex is coagulated
- C. sulphur is chemically combined in the rubber
- D. water is removed from the rubber
When excess chlorine is mixed with ethene at room temperature, the product is
- A. 1,2 - dichloroethane
- B. 1,2 - dichloroethene
- C. 1,1 - dichloroethane
- D. 1, 1 - dichloroethene
Which of the following is NOT involved in the extraction of metals from their ores?
- A. reduction with carbon
- B. reduction with other metals
- C. reduction by electrolysis
- D. oxidation with oxidizing agents
Mortar is NOT used for under-water construction because
- A. it hardens by lose of water
- B. its hardening doe not depend upon evaporation
- C. it requires concrete to harden
- D. it will be washed away by the flow of water
A metal is extracted from its ore by the electrolysis of its molten chloride and it displaces lead from lead (ll) trioxonitrate (V) solution. The metal is
- A. copper
- B. aluminium
- C. zinc
- D. sodium
The scale of a chemical balance is made of iron plate and coated with copper electrolytically because
- A. iron is less susceptible to corrosion than copper
- B. copper is twice as less susceptitible to corrosion as iron
- C. copper is less susceptible to corrosion than iron
- D. copper and iron are equally susceptible to corrosion


