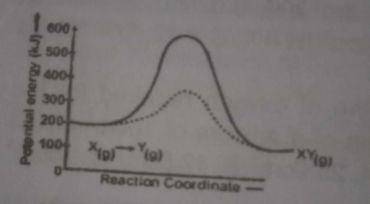
The above diagram gives the potential energy profile of the catalyst and uncatalysed reactions of X(g) + Y(g) → XY(g). Deduce the respective activation energies in KJ of the catalysed and uncatalysed reverse reactions: XY(g) → X(g) + Y(g)
The correct answer is: A
Explanation
Activation energy, E\(_a\) of catalysed reaction is 400 - 100kJ = 300kJ and the activation energy, E\(_a\) of uncatalysed reaction = 600 - 100kJ = 500kJ
or
The catalysed reaction = 100 - 400 = -300
The uncatalysed reverse reaction = 100 - 600 = - 500. From the profile diagram, the forward reaction is an exothermic, ΔH = - ve, - (- 300) = 300kJ
Also, since the forward reaction was exothermic, the reverse reaction will be endothermic, which means ΔH = + ve . Therefore, uncatalysed reversible reaction will be 500kJ.
Consequently, the correct answer is Option A - 300, 500kJ


