The Avogadro number of 24g of magnesium is the same as that of
- A. 1g of hydrogen molecules
- B. 16g of oxygen molecules
- C. 32g of oxygen molecules
- D. 35.5g of chlorine molecules
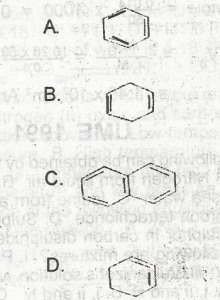
Select the right answer from the structure above.Which of the following compounds represents the polymerization product of ethyne?
- A. A
- B. B
- C. C
- D. D
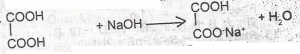
The above reaction is an example of
- A. a displacement reaction
- B. a neutralization reaction
- C. an elimination reaction
- D. saponification

Use the above graph above to answer this question . CH3-C = CH \(\frac{Na}{liq NH_3}\)> P, Compound P, in the above reaction, is
- A. A
- B. B
- C. C
- D. D
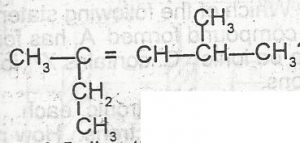
What is the IUPAC name for the hydrocarbon?
- A. 2-ethyl-4-methylpent-2-ene
- B. 3,5-dimethylhex-3-ene
- C. 2,4-dimethylhex 3-ene
- D. 2-methyl-4-ethylpent-3-ene
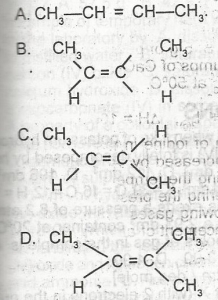
Use the graph above to answer this question. The structure of cis-2-butene is
- A. A
- B. B
- C. C
- D. D
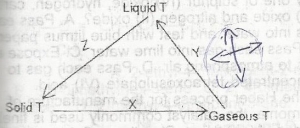
Changes in the physical in the scheme above.The letter X,Y and Z respectively represent
- A. sublimation, condensation and freezing
- B. sublimation, vapourization and solidification
- C. freezing, condensation and sublimation
- D. evaporation, liquefaction and solidification
0.1 faraday of electricity deposited 2.95 g of nickel during electrolysis of an aqueous solution.
Calculate the number of moles of nickel that will be deposited by 0.4 faraday?
(Ni = 58.7)
- A. 0.20
- B. 0.30
- C. 0.04
- D. 5.87
If 10.8 g of silver is deposited in a silver coulometer volume of oxygen liberated is?
- A. 0.56 dm3
- B. 5.60 dm3
- C. 11.20 dm3
- D. 22.40 dm3
What volume of 11.0 M hydrochloric acid must be dilute to obtain 1 dm3 of 0.05 M acid?
- A. 0.05 dm3
- B. 0.10 dm3
- C. 0.55 dm3
- D. 11.0 dm3
What is the concentration of H+ ions in moles per dm3 of a sodium of pH 4.398?
- A. 4.0 χ 10-5
- B. 0.4 χ 10-5
- C. 4.0 χ 10-3
- D. 0.4 χ 10-3
2.0g of a monobasic acid was made up to 250 cm3 with distilled water. 25.00 cm3 of this solution required 20.00cm3 of 0.1 M NaOH solution for complete neutralization. The molar mass of the acid is?
- A. 200 g
- B. 160 g
- C. 100 g
- D. 50 g
Na2C2O4 + CaCl2 → CaC2O4 + 2NaCl. Given a solution of 1.9g of sodium oxalate in 50g of water at room temperature, calculate the minimum volume of 0.1 M calcium oxalate using the above equation?
- A. 1.40 χ 102 dm3
- B. 14.0 χ 102 cm3
- C. 1.40 χ 10-2 dm-2
- D. 14.0 χ 10-2 cm3
Smoke consist of?
- A. solid particles dispersed in liquid
- B. solid or liquid particles dispersed in gas
- C. gas or liquid particles dispersed inliquid
- D. liquid particles dispersed inliquid
The solubility in moles per dm3 of 20g of CuSO4 dissolve in 100 of water at 180°C is?
(Cu = 63.5, S = 32, O = 16)
- A. 0.13
- B. 0.25
- C. 1.25
- D. 2.00
A blue solid, T, which weighed 5.0g was placed on a table. After 8 hours, the resulting pink solid was found to weigh 5.5g. It can be inferred that substance T?
- A. is deliquescent
- B. is hydroscopic
- C. has some molecules of water of crystallization
- D. is efflorescent
10.0 dm3 of air containing H2S as an impurity was passed through a solution of Pb(NO3)2 until all the H2S had reacted. The precipitate of PbS was found to weigh 5.02 g. According to the equation:
Pb(NO3)2 + H2S → PbS + 2HNO3 the percentage by volume of hydrogen sulphide in the air is?
(Pb = 207, S = 32, GMV at s.t.p = 22.4dm3)
- A. 50.2
- B. 47.0
- C. 4.70
- D. 0.47
Chlorine, consisting of two isotopes of mass numbers 35 and 37, has an atomic mass of 35.5.
The relative abundance of the isotope of mass number 37 is?
- A. 20
- B. 25
- C. 50
- D. 75
X(g) → X\(^-\)(g). The type of energy involved in the above transformation is?
- A. ionization energy
- B. sublimation energy
- C. lattice energy
- D. electron affinity
Which of the following terms indicates the number of bonds that can be formed by an atom?
- A. Oxidation number
- B. Valence
- C. Atomic number
- D. Electronegativity
…………………P….Q…..R……S
Proton…………..13….16….17…..19
Electron…………13….16….17…..19
Neutron………….14….16….35…..20
Which of the four atoms P,q,R and in S in the above data can be described by the following properties relative atomic mass is greater than 30 but less than 40; it has an odd atomic number and forms a unipositive ion in solution?
- A. P
- B. Q
- C. R
- D. S


