The fraction of crude oil used as jet fuel is
- A. refinery gas
- B. diesel oil
- C. kerosine
- D. gasoline
At 25oC and 1atm, a gas occupies a volume of 1.50dm3. What volume will it occupy at 100oC and 1atm?
- A. 1.88 dm3
- B. 6.00 dm3
- C. 18.80 dm3
- D. 18.80 dm3
- E. 60.00dm3
The dissolution of common salt in water is a physical change because
- A. the salt can be obtained by crystallization
- B. the salt can be recovered by the evaporation of water
- C. heat is not generated during mixing
- D. the solution will not boil at 100oC
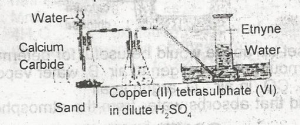
The function of the copper (ll)tetraoxosulphate (IV) in dilute H2SO4 in the figure above is to
- A. dry the gas
- B. absorb phosphine impurity
- C. absorb ethene impurity
- D. from an acetylide with ethyne
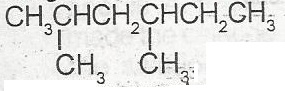
The IUPAC nomenclature for the compound above is
- A. dimethlhexane
- B. 3,5 dimethylhexane
- C. 1.1 dmethyl, 3 methylpentane
- D. 2.4 dimethylhexane

The two functional groups in the above compound are
- A. alcohol and amine
- B. acid and amine
- C. aldehyde and acid
- D. ketone and amine
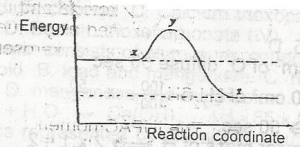
In the diagram above, curve X represents the energy profile for a homogeneous gaseous reaction. Which of the following conditions would produce curve Y for the same reaction?
- A. Increase in temperature
- B. Increase in the concentration of a reactant
- C. Addition of a catalyst
- D. Increase in pressure

Half-cell reaction. From the data above , it can be deduced that the most powerful reducing agent of the four metals is
- A. Cu
- B. Fe
- C. Ba
- D. Zn
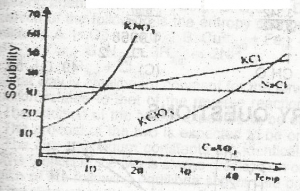
For which salt in the graph above does the solubility increase most rapidly with rise in temperature?
- A. CaSO4
- B. KNO3
- C. NaCl
- D. KCl
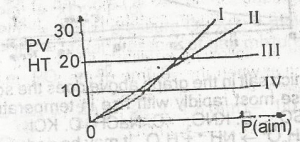
Which of the curves above represents the behaviour of 1 mole of an ideal gas?
- A. l
- B. ll
- C. lll
- D. lV
A gas that will turn orange potassium heptaoxodichromate (lV) solution to clear green is?
- A. sulphur (ii) oxide
- B. hydrogen sulphide
- C. sulphide (lV) oxide
- D. hydrogen chloride
The confirmatory test for alkanoic acids in organic qualitative analysis is the?
- A. turning of wet blue litmus paper red
- B. reaction with alkanols to form esters
- C. reaction with sodium hydroxide to form salt and water
- D. reaction with aqueous Na2COsp3 to liberate a gas which turns lime water milky
Which of the following represents saponification?
- A. Reaction of carboxylic acids with sodium hydroxide
- B. Reaction of alkanoates with acid
- C. Reaction of alkanoates with alcohols
- D. Reaction of alkanoates with sodium hydroxide
Catalytic hydrogenation of benzene produces?
- A. an aromatic hydrocarbon
- B. margarine
- C. cyclohexane
- D. D.D.T
Palm wine turns sour with time because?
- A. the sugar content is converted into alcohol
- B. the carbon (IV) oxide formed during the fermentation process has a sour taste
- C. it is commonly adulterated by the tappers and sellers
- D. microbial activity results in the production of orgain acids within it
The carbon atoms on ethane are?
- A. sp2 hybridized
- B. sp3 hybridized
- C. sp2d hybridized
- D. sp hybridized
Al2O3(s) + 3H2SO4(aq) → Al2(SO4(aq)) + 3H2(I)O
Al2O3(s) + 2NaOH(aq)) + 3H2(I)O → 2NaAl(OH)4(aq)).
We can conclude from the equations above that Al2O3(s) is?
- A. an acidic oxide
- B. an amphoteric oxide
- C. a basic oxide
- D. a neutral oxide
It is not desirable to use lead tetraethyl as an anti-knock agent because?
- A. it is expensive
- B. of pollution effects from the exhaust fumes
- C. it lowers the octane rating of petrol
- D. it is explosive
The fraction of crude oil used as jet fuel is?
- A. refinery gas
- B. diesel oil
- C. kerosene
- D. gasoline
The active reducing agent in the blast furnace for the extraction of iron is?
- A. carbon
- B. limestone
- C. carbon(II)oxide
- D. calcium oxide
Bronze is an alloy of?
- A. silver and copper
- B. silver and gold
- C. copper and nickle
- D. copper and zinc
- E. copper and tin


