
From the above diagram; The caustic soda in the conical flask serves to
- A. dry ethene gas
- B. remove carbon (iv) oxides from ethene
- C. remove carbon (ii) oxide from ethene
- D. remove sulphur (iv) oxide from ethene
When sodium ethanoate is treated with a few drops of concentrated tetraoxosulphate(vi)acid. one of the products is
- A. CH3COOH
- B. CH3COOCH3
- C. CH3COOC2H5
- D. C2H5COOCH3
Clothes should be properly rinsed with water after bleaching because
- A. the bleach decolourizes the clothes
- B. chlorine reacts with fabrics during bleaching
- C. the clothes are sterilized during bleaching
- D. hydrogen chloride solution is produced during bleaching
which of these metals CANNOT replace hydrogen from alkaline solutions?
- A. Aluminium
- B. Zinc
- C. Tin
- D. Iron
MnO4(aq) + 8H + 5Fe2+ \(\to\) Mn2+(aq) + 5Fe3+(aq) + H2O. The oxidation number of maganese in the above reaction changed from
- A. +7 to +2
- B. +6 to +2
- C. +5 to +2
- D. +4 to +2
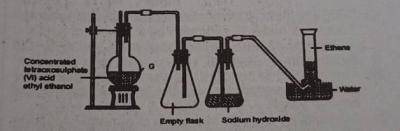
Use the diagram above to answer this question. The reaction taking place in flask G is known as?
- A. hydrolysis
- B. double decomposition
- C. dehydration
- D. pyrolysis
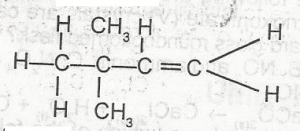
The IUPAC name of the compound above is
- A. 2,2-dimethyl but -1-yne
- B. 2,2-dmethyl but -1-ene
- C. 3,3-dimethyl but -1-ene
- D. 3,3-dimethyl but-1-yne
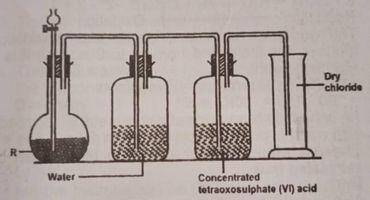
In the diagram above, R is a mixture of?
- A. potassium tetraxochlorate (VII) and concentrated H2SO4
- B. potassium trioxochlorate (V) and concentrated H2SO4
- C. potassium tetratoxomanganate (VII) and concentrated HCI
- D. manganese (IV) oxide and concentrated HCI
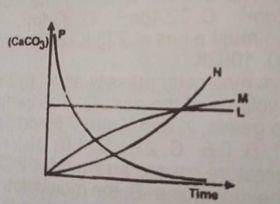
2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from the reaction above, which of the following curves represents the consumption of calcium trioxocarbonate (IV) as dilute HCL is added to it?
- A. L
- B. M
- C. N
- D. P
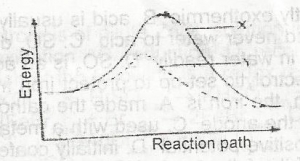
In the diagram above the activation energy is represented by
- A. y - x
- B. x
- C. x - z
- D. y
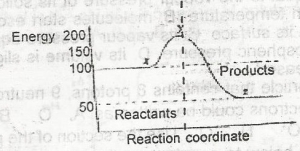
The ∆H for the reaction represented by the energy profile above is
- A. -100 kJ mol--1
- B. + 100 kJ mol-1
- C. +50 kJ mol-1
- D. -50 kJ mol-1
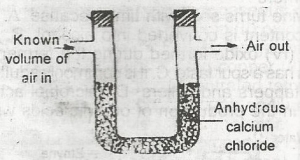
The set-up above would be useful for determining the amount of
- A. oxygen in air
- B. water vapour in air
- C. CO2 in air
- D. arygen in air
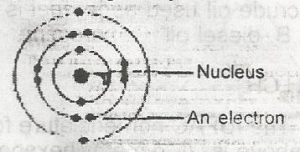
The diagram above represents an atom of
- A. magnesium
- B. helium
- C. chlorine
- D. neon
Which of the following reagents will confirm the presence of unsaturation in a compound?
- A. Fehling's solution
- B. Bromine water
- C. Tollen's reagent
- D. Benedict's solution
One mole of a hydrocarbon contains 48 g of carbon. If it vapour density is 28, the hydrocarbon is?
- A. an alkane
- B. an alkene
- C. an alkyne
- D. aromatic (C = 12, H = 1)
The general formula of alkanoates is?
- A. RCHO
- B. R2CO
- C. RCOOH
- D. RCOOR
When sodium is added to ethanol, the products are?
- A. sodium hydroxide and water
- B. sodium hydroxide and hydrogen
- C. sodium ethoxide and water
- D. sodium ethoxide and hydrogen
Which of the following compounds is NOT an isomers of 2,2 dimethylbutane?
- A. 2-methylbutane
- B. 3-methylpentane
- C. 2,3-dimethylbutane
- D. 2-methylpentane
Which of the following is the correct order of decreasing activity of the metals Fe, Ca, Al and Na?
- A. Fe> Ca> Al> Na
- B. Na> Ca> Al> Fe
- C. Al> Fe> Na> Ca
- D. Ca> Na> Fe>, Al
Which of the following is NOT true of metals?
- A. They are good conductors of electricity
- B. They ionize by electron loss
- C. Their oxides are acidic
- D. They have high melting points
In an electrolytic set-up to protect iron from corrosion, the iron is?
- A. made cathode
- B. made anode
- C. used with a metal of lower electropositive potential
- D. initially coated with tin


