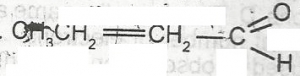
The functional group represented in the compound above is?
- A. alkanol
- B. alkanal
- C. alkanone
- D. alanoate
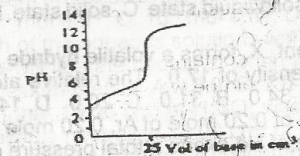
The option above shows the PH changes for the titration of a
- A. strong acid versus strong base
- B. weak acid versus strong base
- C. strong acid versus weak base
- D. weak acid versus weak base
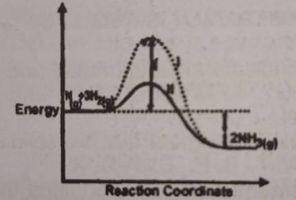
It can be deduced that the rate of the reaction?
- A. for bath l is higher than path ll
- B. for path ll is higher than path l
- C. is the same for both paths at all temperatures
- D. depends on the values of both x and y at all pressures
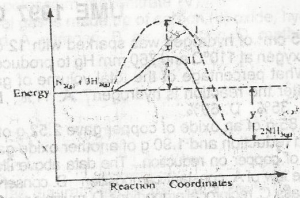
Use the diagram above to answer this question. The activation energy of the uncatalysed reaction is
- A. X
- B. X + Y
- C. X - Y
- D. Y
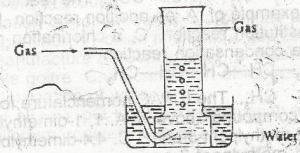
The arrangement above can be used for the collection of
- A. sulphur (IV) oxide
- B. ammonia
- C. hydrogen chloride
- D. nitrogen

USE THE SECTION OF THE PERIODIC TABLE ABOVE TO ANSWER THIS QUESTION.Which letter represents a non-metal that is a solid at room temperature?
- A. T
- B. R
- C. J
- D. X

USE THE SECTION OF THE PERIODIC TABLE ABOVE TO ANSWER THIS QUESTION.Which of the indicate an alkali metal and a noble gas respectively?
- A. M and E
- B. G and E
- C. R and L
- D. G and L
The relatively high boiling points of alkanols are due to?
- A. ionic bonding
- B. aromatic character
- C. covalent bonding
- D. hydrogen bonding
An example of a secondary amine is?
- A. propylene
- B. di-butylamine
- C. methylamine
- D. trimethylamine
CxHY + 4O2 → 3CO2 + 2H2O. The hydrocarbon, CxHY, in the reaction above is?
- A. propane
- B. propene
- C. propyne
- D. propanone
The role of sodium chloride in the preparation of soap is to?
- A. purify the soap
- B. separate the soap from glycerol
- C. accelerate the decomposition of the fat or oil
- D. react with glycerol
Aromatic and aliphatic hydrocarbons can be distinguished from each other by the?
- A. action of bromine
- B. use of polymerization reaction
- C. action of heat
- D. use of oxidation reaction
Which of the following pairs has compounds that are isomers?
- A. Propanal and propanone
- B. Ethanoic acid and ethylmethanoate
- C. Ethanoic acid and ethane-1, 2-diol
- D. 2-methylbutane and 2, 2-dimethylbutane
CHCl3 + Cl2 → HCl + CCl4. The reaction above is an example of?
- A. an addition reaction
- B. a substitution reaction
- C. a chlorination reaction
- D. a condensation reaction
Group 1 A metals are not found free in nature because they?
- A. are of low melting and boiling points
- B. have weak metallic bonding
- C. conduct electricity and heat
- D. are very reactive
Which of the following compounds will impart a brick-red colour to a non-luminious bunsen flame?
- A. NaCl
- B. LiCl
- C. CaCl2
- D. MgCl2
In the extraction of iron in the blast furnace, limestone is used to?
- A. release CO2 for the reaction
- B. reduce the iron ore
- C. increase the strenght of the iron
- D. remove impurities
Which of the following ions will give a white precipitate with aqueous NaOH and soluble in excess of the base?
- A. Ca2+
- B. Mg2+
- C. Zn2+
- D. Cu2+
A gas that will turn orange potassium heptaoxodichromate (lV) solution to clear green is?
- A. sulphur (lV) oxide
- B. hydrogen sulphide
- C. sulphide (lV) oxide
- D. hydrogen chloride
Sulphur exists in six forms in the solid state. This property is known as?
- A. isomerism
- B. allotropy
- C. isotopy
- D. isomorphism
In the industrial production of hydrogen from natural gas, carbon (IV) oxide produced along with the hydrogen is removed by?
- A. washing with water under pressure
- B. passing the mixture into lime water
- C. using ammoniacal copper (l) chloride
- D. drying over phoshorus (V) oxide


