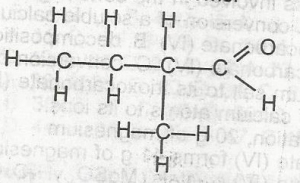
The compound above is the product of the oxidation of
- A. 2-methlbutan-2-ol
- B. 2-methylbutan -1- ol
- C. 2, 3-dimethylprop 1-ol
- D. Pentan -2- ol
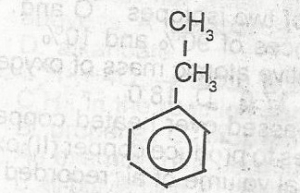
The compound above contains
- A. Sp 3 hybridized carbon atoms only
- B. Sp 2 hybridized carbon atoms only
- C. Sp 3 and Sp hybirdized carbon atoms
- D. Sp 3 and Sp 2
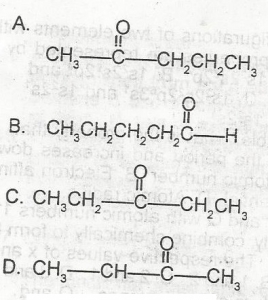
Choose the correct answer from the options above. When Fehling is solution is added to two isomeric carbonyl compounds X and Y with the molecular formula C 5H 10O, compound X gives a red precipitate while Y does not react. It can be inferred that X is
- A. A
- B. B
- C. C
- D. D
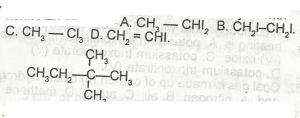
How many more isomers of the compound above can be obtained ?
- A. 5
- B. 4
- C. 3
- D. 2
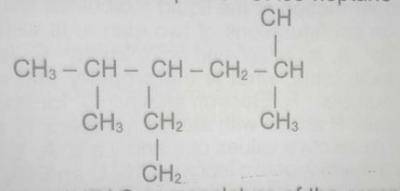
The IUPAC nomenclature of the organic compound with the above structural formula is
- A. 3-ethyl-2,5-dimethylhexane
- B. 4-ethyl-2,5-dimethylhexane
- C. 3-ethyl-2,5,5 - trimethylpentane
- D. 3-ethyl, 1,1,4-trimethylpentane
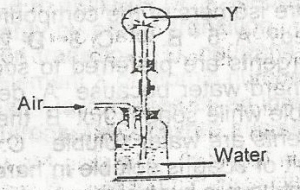
In the diagram above the gas Y could be
- A. hydrogen chloride
- B. oxygen
- C. carbon (IV) oxide
- D. chlorine
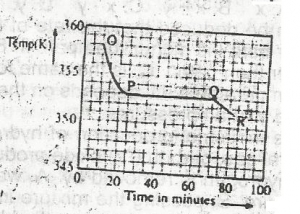
Use the graph above to answer this question. A sample, X, solid at room temperature, was melted, heated to a temperature of 358 K and allowed to cool as shown in OPQR.The section OP suggests that X is in the
- A. liquid sate
- B. solid/liquid state
- C. solid state
- D. gaseous state
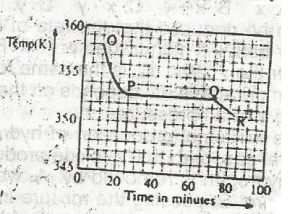
Use the graph above to answer this question. A sample, X, solid at room temperature, was melted, heated to a temperature of 358 K and allowed to cool as shown in OPQR. The section PQ indicates that X is
- A. a mixture of salts
- B. a hydrated salt
- C. an ionic salt
- D. a pure compound
(1 /(2) N2(g) + (1) / (2)O2(g) → NO(g) ∆H° 89 KJ mol–. If the entropy change for the reaction above at 25°C is 11.8 J mol–, calculate the change in free energy. ∆G°, for the reaction at 25°C?
- A. 88.71 KJ
- B. 85.48 KJ
- C. -204.00 KJ
- D. -3427.40 KJ
25 cm3 of 0.02 M KOH neutralized 0.03 g of a monobasic organic acid having the general formula CnH2n + 1 COOH. The molecular formula of the acid is?
(C = 12, H = 1, O = 16)
- A. HCOOH
- B. C2H5COOH
- C. CH3COOH
- D. C3H7COOH
The synthetic detergents are preferred to soap for laundry using hard water because?
- A. detergents are water soluble while soap is not
- B. the calcium salts of detergents are water soluble
- C. the magnesium salt of soap is soluble in hard water
- D. soap does not have a hydrocarbon terminal chain
Synthetic rubber obtained by the polymerization of chlorobutadiene in the presence of sodium is called?
- A. teflon
- B. isoprene
- C. polythene
- D. neoprene
The final product of the reaction of ethyne with hydrogen iodine is?
- A. CH3—CHI2
- B. CH2l—CH2I
- C. CH3—CI3
- D. CH2 = CHI
The reaction of an alkanol with an alkanoic acid in the presence of concentrated H2SO4 will produce an?
- A. alkanal
- B. alkanoate
- C. alkanone
- D. alkyne
An undesirable paraffin in the petroleum industry which is particularly prone to knocking is?
- A. iso-octane
- B. n-heptane
- C. iso-heptane
- D. n-octane
A sample of a substance containing only C and H burns in excess O2 to yield 4.4g of CO2
and 2.7 g of H2O. The empirical formula of a substance is?
(C = 12, O = 16, H = 1)
- A. CH3
- B. CH2
- C. CH4
- D. C2H24
Sodium hydroxide is prepared commercially from sodium chloride solution by?
- A. electrolysis using mercury as cathode
- B. hydrolysis in steam using a catalyst
- C. electrolysis using iron as anode
- D. treating sodium chloride with ammonia and carbon (IV) oxide
Which of the additives could improve the quality of steel?
- A. Silicon
- B. Sulphur and phosphorus
- C. Carbon
- D. Chromium and nickel
The removal of rust from iron by treatment with tetraoxosulphate (IV) acid is based on the?
- A. hydrolysis of the iron
- B. reaction of acid with base
- C. oxidationof the rust
- D. dehydration of the iron
2X(aq) + MnO2(s) + 4H+(aq) → X2(g) + Mn2+(aq) + 2H2O.
The reaction above can be used for the laboratory preparation of all halogens except fluorine because it is?
- A. a poisionous gas
- B. an oxidizing agent
- C. electronegative in nature
- D. highly reactive
Coal gas is made up of carbon (II) oxide, hydrogen and?
- A. nitrogen
- B. air
- C. argon
- D. methane


