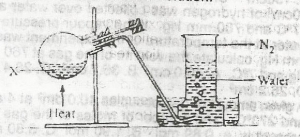
In the experiment above, X could be a solution of
- A. sodium trioxonitrate (V) and ammonium chloride
- B. sodium trioxonitrate (lll) and ammonium chloride
- C. lead (ll) trioxonitrate (V) and copper turnings
- D. potassium trioxonitrate (V) and copper turnings
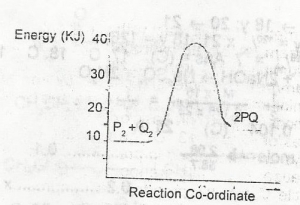
In the diagram above , the activation energy for the backward reaction is
- A. +5kJ
- B. +15KJ
- C. +25KJ
- D. +30KJ

The diagram above represents an atom that can combine with chlorine to form
- A. a covalent bond
- B. an electrovalent bond
- C. a hydrogen bond
- D. a co-ordinate bond
Which of the following is true concerning the properties of benzene and hexane?
- A. Both undergo substitution reaction
- B. Both undergo addition reaction
- C. Both are solids
- D. Both can decolourized bromine water
Terylene is synthesized from ethane- 1, 2-diol and benzene – 1, 4-dicarboxylic acid by?
- A. addition reaction
- B. condensation reaction
- C. elimination reaction
- D. substitution reaction
An example of aromatic compound is?
- A. C6H13OH
- B. C6H13CI
- C. C6H5OH
- D. C6H14
How many structural isomers can be drawn for the non-cyclic alkanol with molecular formula C 4H10O?
- A. 1
- B. 2
- C. 3
- D. 4
On cracking medicinal paraffin, a gas is evolved which gives a pop sound with a lighted splinter and an oily liquid which decolourizes bromine solution is also obtained. The products of the cracking are ?
- A. carbon (IV) oxide and alkyne
- B. carbon (ll) oxide and alkene
- C. hydrogen gas and alkene
- D. hydrogen gas and alkane
One mole of hydrocarbon contains 6g of hydrogen. If the molecular weight is 54, the hydrocarbon is an?
- A. alkanone
- B. alkane
- C. alkene
- D. alkyne
Alloys are often used in preference to pure metals because?
- A. metals are too hard
- B. merals are ductile
- C. metallic properties are improve in alloys
- D. alloys are a mixture of metals
A common characteristic shared by iron and aluminium is that both?
- A. are extracted by reduction methods
- B. form only basic oxides
- C. show oxidation states of +2 and +3
- D. form soluble hydroxides
The metal that will react with water only in the form of steam to librate hydrogen gas is?
- A. calcium
- B. aluminium
- C. iron
- D. zinc
Which of the following is observed when a solution of iron (III) chloride is mixed with a solution of sodium hydroxide?
- A. A gas is evolved
- B. A deep blue precipitate is formed
- C. The resultant solution turns green turns
- D. A brown precipitate is formed
The oxide that remains unchanged when heated in hydrogen is?
- A. CuO
- B. Fe2O3
- C. PbO2
- D. ZnO
A solution containing chloride ions gives white precipitate with silver trioxonitrate(V) Solution. The precipitate will be insoluble in dilute?
- A. HNO 3 but soluble in ammonia solution
- B. HNO3 and ammonia solution
- C. HCl but soluble in ammonia solution
- D. HCl and in ammonia solution
3CuO(s) + 2NH3(g) → 3Cu(s) + 3H2O(I) N2(g) (i).
2NH3(g) + 3Cl2(g) → 6HCl(g) + N2(g) (ii).
4NH3(g) + 3O2(g) → 6H2O(I) + 2N2(g) (iii).
The reactions represented by the equations above demonstrate the?
- A. basic properties of ammonia
- B. acidic properties of ammonia
- C. reducing properties of ammonia
- D. oxidizing properties of ammonia
A gas that turns a filter paper previously soaked in lead ethanoate solution black is?
- A. hyrogen chloride
- B. hydrogen sulphide
- C. sulphur (IV) oxide
- D. sulphur (VI) oxide
2X(g) + Y(g) → Z(g): In the equation above, The rate of formation of Z is found to be independent of the concentration of Y and to quadruple when the concentration of X is doubled. The correct equation for the reaction is?
- A. R = k (X)(Y)
- B. R = K (X) ° (Y)
- C. R = K (X) ° (Y) 2
- D. R = K (X) 2(Y)°
2Cl2(g) + 2H2O(g) 4HCl(g) + O2(g)
∆H° = +115KJ mol-1. In the above equilibrium reaction, a decrease in temperature will?
- A. favour the reverse reaction
- B. favour the forward reaction
- C. have no effect on the equilibrium state
- D. double the rate of the reverse raection
Mg2+(aq) + 2e–>(aq) → Mg(s)E°(volts) = 2.370
Zn2+(aq) + 2e–>(aq) → Zn(s)E°(volts) = -0.763
Cds2+(aq) + 2e–>(aq) → Cd(s)E°(volts) = 0.403
Cu2+(aq) + 2e–>(aq) → Cu(s)E°(volts) = 0.340
In the electrochemical series above, the strongest reducing agents is?
- A. Cu (s)
- B. Cd(s)
- C. Znsub>(s)
- D. Mg(s)
The pH of a solution obtained by mixing 100cm3 of a 0.2M solution of NaOH is?
- A. 1.3
- B. 7.0
- C. 9.7
- D. 12.7


