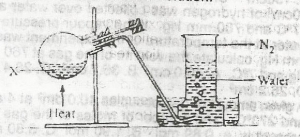
In the experiment above, X could be a solution of
The correct answer is: B
Explanation
Nitrogen is prepared in the laboratory by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. The ammonium nitrite formed as a result of a double decomposition reaction, decomposes to form nitrogen gas.
\(NH_4Cl_{(aq)}+NaNO_{2(aq)} → NH_4NO_{2(aq)}+NaCl_{(aq)}\)
\(NH_4NO_{2(aq)} →N_{2(g)} +2H_2O_{(l)}\).
(source: understanding chemistry by G O Ojukuku)


