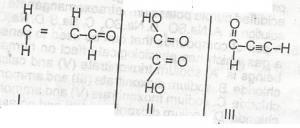
Which of the compounds above would react to take up two molecules of bromine durng bromination?
- A. l only
- B. lll only
- C. l and ll only
- D. ll and lll only
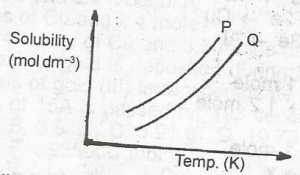
In the diagram above, the mixture of the solids P and Q can be separated by?
- A. distillation
- B. fractional distillation
- C. crystallization
- D. fractional crystallization
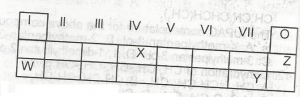
Use the table above to answer this question .The least reactive element is
- A. W
- B. X
- C. Y
- D. Z

Use the table above to answer this question . The element that is likely to participate in covalent rather than ionic bonding is
- A. Z
- B. Y
- C. X
- D. W
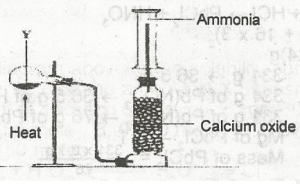
In the diagram above, Y is a mixture of
- A. calcium hydroxide and ammonium chloride
- B. calcuim hydroxide and sodium chloride
- C. sodium chloride and ammonuim trioxonitrate (V)
- D. sodium dioxonitrate (lll) and ammouium chloride
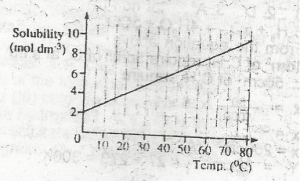
From the diagram above the mass of crystals deposited when 1 dm 3 of a saturated solution of NaCl is cooled from 80 oC to 60oC is
[Na = 23, Cl = 35.5]
- A. 117.00 g
- B. 58.50 g
- C. 11.70 g
- D. 5.85 g
When the two end alkyl groups ethyl ethanoate are interchange, the compound formed is known as
- A. methyl ethanoate
- B. ethyl propanaote
- C. methyl propanoate
- D. propyl ethanoate
An organic compound that does not undergo a reaction with both hydrogen cyanide and hydroxylamine can be
- A. alkene
- B. alkanal
- C. alkanone
- D. alkanoic acid
POlyvinyl chloride is used to produce
- A. bread
- B. pencils
- C. ink
- D. pipes
When excess ethanol is heated to 145oC in the presence of concentrated H2SO4, the product is
- A. ethyne
- B. diethyl sulphate
- C. diethyl ether
- D. acetone
Which of the following is a solvent for perfumes?
- A. C5H12
- B. C4H6
- C. CH3COOH
- D. C2H5OH
2-methylprop-1-ene is an isomer of
- A. but-2-ene
- B. pent-1-ene
- C. 2-methylbut-2-ene
- D. 2-methylbut-1-ene
The hydrocarbon that burns in air with a sooty flames is
- A. C2H6
- B. C3H8
- C. C4H10
- D. C6H6
Which of the following metals is passive to concentrated trioxonitrate (V) acid?
- A. Iron
- B. Tin
- C. Copper
- D. Zinc
A metal that can be extracted from cassitertite is
- A. cacium
- B. magnesium
- C. tin
- D. copper
The pair of metals in the reactivity series that are usually extracted by the electrolysis of their ores is
- A. magnesium and zinc
- B. magnesium and calcium
- C. copper and zinc
- D. lead and calcium
What properties of duralumin make it more useful than its constituent metals?
- A. It is heavy with a high melting point
- B. It is malleable and has high density
- C. It is strong and light
- D. It is hard and ductile
Hydrogen is used in oxy-hydrogen flames for melting metals because it
- A. evolves a lot of heat when burnt
- B. combines explosively with oxygen
- C. is a very light gas
- D. is a rocket fuel
A pair of compounds that can be used to generate a gas which has physiological effect on human beings is
- A. sodium trioxonitrate (V) and calcium chloride
- B. sodium dioxonitrate (III) and ammonium chloride
- C. sodium trioxonitrate (V) and ammonium chloride
- D. sodium dioxonitrate (III) and potassium chloride
The salt that reacts with dilute hydrochloric acid to produce a pungent smelling gas which decolourizes acidified purple potassium tetraoxomanganate (VII) solution is
- A. Na2SO4
- B. Na2SO3
- C. Na2S
- D. Na2CO3
Chlorine, bromine and iodine resemble one another in that they?
- A. dissolve in alkalis
- B. react violently with hydrogen without heating
- C. are liquids
- D. displace one another from solutions of their salts


