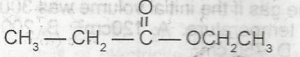
Use the option above to answer this question. The graph that describes a zero order reaction is
- A. A
- B. B
- C. C
- D. D
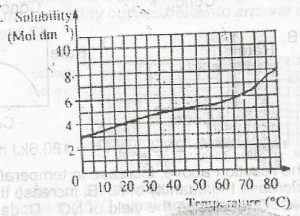
The diagram above is the solubility curve of a solute, X. Find the amount of X deposited when 500cm3 of a solution of X is cooled from 60oC to 20oC.
- A. 0.750 mole
- B. 0.950 mole
- C. 2.375 moles
- D. 4.750 moles
Sucrose is made up of
- A. glucose and glucose
- B. glucose and fructose
- C. fructose and fructose
- D. galactose and glucose
Alkanones are generally obtained by the oxidation of
- A. primary alkanols
- B. secondary alkanols
- C. tertiary alkanols
- D. alkanoic acid
The reaction of carbide with water gives
- A. ethyne
- B. ethene
- C. ethane
- D. ethanal
The chlorinated alkane often used industrially to remove grease is
- A. tetrachloromethane
- B. chloromethane
- C. trichloromethane
- D. dichlorometane
The conditions necessary for the extraction of a water molecule from two molecules of ethanol are
- A. less acid and lower temperature
- B. excess acid and a lower temperature
- C. excess acid and a higher temperature
- D. less acid and a higher temperature
Unsaturated organic compounds are identified by decolourization of
- A. silver bromine and potassium tetraoxomanganate (VII) solutions
- B. bromine water and acidified potassium tetraoxomanganate (VII) solution
- C. silver bromide solution and bromine water
- D. bromine water and alkaline potassium tetraoxomanganate (VII) solution
The repeating unit in natural rubber is
- A. alkyne
- B. isoprene
- C. n-propene
- D. peoprene
The least easily oxidized of the metals below is?
- A. Ca
- B. Na
- C. Zn
- D. Al
A common characteristics of copper and silver in their usage as coinage metals is that they
- A. have high metallic lustre
- B. are not easily oxidized
- C. are easily oxidized
- D. are not easily reduced
Potassium vapour burns with a
- A. blue flame
- B. brick-red flame
- C. violet flame
- D. golden-yellow flame
Synthesis gas is a mixture of
- A. CH4 and H2O
- B. CH4 and H2
- C. CO2 and H2
- D. CO and H2
A form of carbon used for absorbing poisonous gases and purification of noble gases is
- A. wood charcoal
- B. animal charcoal
- C. carbon fibres
- D. carbon black
The refreshing and characteristic taste of soda water and other soft drinks is as a result of the presence in them of
- A. carbon (IV) oxide
- B. carbon (II) oxide
- C. soda
- D. glucose
A gas that is not associated with global warming is
- A. CO2
- B. SO3
- C. CH4
- D. H2
The substance often used for vulcanization of rubber is
- A. chlorine
- B. hydrogen peroxide
- C. sulphur
- D. tetraoxosulphate (IV) acid
A phenomenon where an element exist in different forms in the same physical state is known as
- A. isomerism
- B. amorphism
- C. allotropy
- D. isotopy
For a reaction in equilibrium, the species involved in the equilibrium constant expression are
- A. gaseous and solid species
- B. liquid and solid species
- C. solid and dissolved species
- D. gaseous and dissolved species