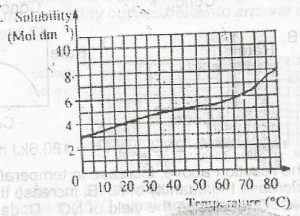
The diagram above is the solubility curve of a solute, X. Find the amount of X deposited when 500cm3 of a solution of X is cooled from 60oC to 20oC.
The correct answer is: A
Explanation
From the graph, when you extrapolate when the temperature is @ 60 deg, Solubility = 5.5 mol/dm3
Also, when the temperature is @ 20 deg, Solubility = 4.0 mol/dm3.
Difference in solubility = 5.5 - 4.0 = 1.5 mol/dm3
Recall that, solubility = amount x 1000/ volume of solvent
1.5 = n x 1000/500
n = 1.5/2
n = 0.750 mole - Option A
There is an explanation video available .


