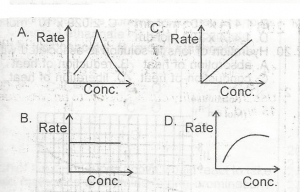
Use the option above to answer this question. The graph that describes a zero order reaction is
The correct answer is: B
Explanation
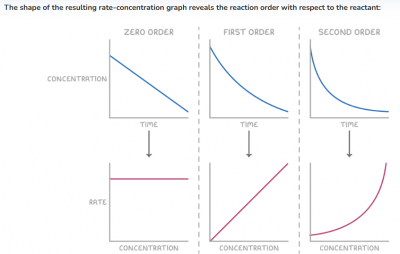
- Zero order - A horizontal line indicates that the reaction rate is independent of the reactant concentration.
The rate equation is: rate = k
- First order - A straight line passing through the origin signifies that the reaction rate is directly proportional to the reactant concentration.
The rate equation is: rate = k[X]
- Second order - A curved plot indicates that the reaction rate is proportional to the square of the reactant concentration.
The rate equation is: rate = k[X]2
There is an explanation video available .


