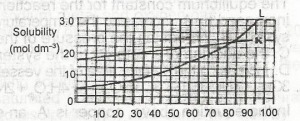
Use the solubility curves below to answer the question. If 1 dm\(^3\) of a saturated solution of L at 60\(^0\)C is cooled to 25\(^0\)C, what amount in mole will separate out?
- A. 0.75
- B. 0.25
- C. 1.00
- D. 0.50
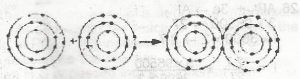
The diagram above represents the formation of
- A. a metallic bond
- B. an electrovalent bond
- C. a covalent bond
- D. a coordinate covalent bond
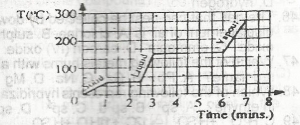
Use the graph above to answer this question.How long does it take all the solid to melt?`
- A. 2.5 mins
- B. 6.0 mins
- C. 1.0 min
- D. 3.0 mins
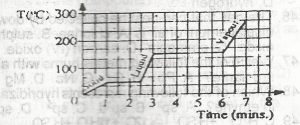
Use the graph above to answer this question.If the gas is cooled, at what temperature will it start to condense?
- A. 125 oC
- B. 150oC
- C. 175oC
- D. 250oC
Which of the following gases contains the least number of atoms at s.t.p?
- A. 1 mole of butane
- B. 3 mole of ozone
- C. 4 mole of chlorine
- D. 7 mole of argon
When sodium reacts with water, the resulting solution is
- A. Weakly acidic
- B. Neutral
- C. Acidic
- D. Alkaline
\(C_{12}H_{22}O_{11(s)} + H_2SO_{4(aq)} → 12C(s) + 11H_2O(l) + H_2SO_{4(aq)}\)
In the reaction above, tetraoxosulphate (VI) acid function as
- A. A dehydrating agent
- B. An oxidizing agent
- C. A reducing agent
- D. A catalyst
Which of the following represent hybridization in ethyne?
- A. Sp2
- B. Sp2d
- C. Sp3
- D. Sp
Which of the following metals burn with brick red flame?
- A. Pb
- B. Ca
- C. Na
- D. Mg
The gas that can best be collected by downward displacement of air is
- A. Chlorine
- B. Sulphur (IV) oxide
- C. Ammonia
- D. Carbon (IV) oxide
A burning candle produces water and
- A. Carbon (II) oxide
- B. Carbon (IV) oxide
- C. Oxygen
- D. Hydrogen
The pair of organic compounds that are isomers is
- A. Benzene and methylbenzene
- B. Trichloromethane and tetrachloromethane
- C. Ethanol and propane
- D. But-1-ene and but-2-ene
A characteristic reaction of the compounds with the general formula C\(_n\)H\(_{2n}\) is
- A. Esterification
- B. Polymerization
- C. Decarboxylation
- D. Substitution
Proteins in acid solution undergo
- A. Polymerization
- B. Substitution
- C. Fermentation
- D. Hydrolysis
When chlorine Is passed into water and the resulting solution exposed to sunlight, the product formed are
- A. Chlorine gas and hydrogen
- B. Oxygen and oxochlorate (I) acid
- C. Chlorine gas and oxochlorate (I) acid
- D. Hydrochloric acid and oxygen
Catalytic hydrogenation of benzene produces
- A. Oil
- B. Cyclohexene
- C. Cyclohexane
- D. Margarine
During the vulcanization of rubber, sulphur is added to
- A. Break down rubber polymer
- B. Lengthen the chain of rubber
- C. Bind rubber molecules together
- D. Act as a catalyst
The main impurity in iron ore during the extraction of iron is
- A. Silicon (IV) oxide
- B. Carbon (IV) oxide
- C. Calcium trioxosilicate
- D. Sulphur (II) oxide
The general formula for the alkanals is
- A. ROH
- B. R2CO
- C. RCOOR1
- D. RCHO
Fermentation is the
- A. breaking down of carbohydrate to glucose
- B. conversion of sugar to alcohol in the presence of yeast
- C. breaking down of sugar to carbohydrate
- D. conversion of alcohol to sugar in the presence of yeast


