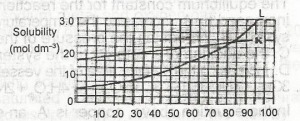
Use the solubility curves below to answer the question. If 1 dm\(^3\) of a saturated solution of L at 60\(^0\)C is cooled to 25\(^0\)C, what amount in mole will separate out?
The correct answer is: A
Explanation
From the solubility curve given, if you extrapolate for solution L at 60\(^0\)C and 25\(^0\)C, the solubility values will be 1.5 moldm\(^{-3}\) and 0.75 moldm\(^{-3}\) respectively.
To determine the amount in mole that will separate out, subtract the solubility of the initial temperature 25\(^0\)C from that of the final temperature 60\(^0\)C.
i.e 1.5 - 0.75 = 0.75 - Option A
There is an explanation video available .


