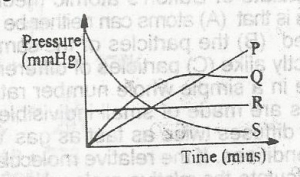
In the diagram above, the curve that represents the production of oxgyen gas from the decomposition of KCIO3 in the presence of MnO2 catalyst is
The correct answer is: A
Explanation
The decomposition of KClO\(_3\) in the presence of MnO\(_2\) catalyst changes solid to gas, which is a sublimation process, as Oxygen gas is produced.
MnO\(_2\)
2KClO\(_3\) → 2KCl + 3O\(_2\)
Decreasing the pressure on a solid causes it to turn into a gas through a process called sublimation. Thus, the effect of pressure on solid is negligible. Consequently, from the curve given in the question, it means pressure is constant as the decomposition reaction proceeds to completion.Therefore, the curve is represented by R.


