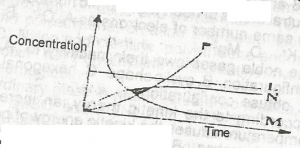
2HCI(ag) + CaCO\(_3\)(s) → CaCl\(_2\)(s) + CO\(_2\)(g) + H\(_2\)O(l). From the reaction above, which of the curves in the diagram represents the production of carbon (IV) oxide as dilute HCI is added?
- A. L
- B. M
- C. N
- D. P
The principal constituent of natural gas is
- A. methane
- B. ethane
- C. propane
- D. butane
Which of the following is found in cotton
- A. Starch
- B. Cellulose
- C. Fat
- D. Oil
The process by which atoms are rearranged into different molecular structure in the petroleum refining process is referred to as
- A. catalytic cracking
- B. hydrocracking
- C. polymerization
- D. reforming
A compound contains 40.0% carbon 6.7% hydrogen and 53.3% oxygen. If the molar mass of the compound is 180, find the molecular formula.
[H = 1, C = 12, O = 16]
- A. CH2O
- B. C3H6O3
- C. C6H12O6
- D. C6H12O3
Ethanol reacts with excess acidified K\(_2\)Cr\(_2\)O\(_7\) to produce
- A. ethanedioic acid
- B. ethanal
- C. ethylethanoate
- D. ethanoic acid
The type of reaction that is peculiar to benzene is?
- A. addition
- B. hydrolysis
- C. polymerization
- D. substitution
The formula for ethyl butanoate is
- A. C3H7COOC2H5
- B. C2H5COOC3H7
- C. C4H9COOC2H5
- D. C2H5COOC4H9
The leachate of a certain plant ash is used in local soap making because it contains
- A. sodium chloride and potassium hydroxide
- B. sodium hydroxide
- C. potassium hydroxide
- D. soluble carbonates and hydrogen carbonates
Ethene reacts with hydrogen bromide to give
- A. CH2Br2
- B. CH3CH2Br
- C. C2H2Br2
- D. CHBr2
The modern process of manufacturing steel for iron is by
- A. treatment with acids
- B. oxidation
- C. blast reduction
- D. treatment with alkanal
Chlorine gas turns a damp starch-iodide paper
- A. pink
- B. colourless
- C. red
- D. dark blue
The metal that liberates hydrogen from cold water in bubbles only is?
- A. Na
- B. K
- C. Ca
- D. Al
The salt that will form a precipitate soluble in excess ammonia solution is
- A. Ca(NO3)2
- B. Cu(NO3)2
- C. Mg(NO3)2
- D. Al(NO3)3
Which of the following statements is true of sulphur (IV) oxide?
- A. It forms tetraoxosulphate (VI) acid with water
- B. It is an odourless gas
- C. It is an acid hydride
- D. It forms white precipitate with acidified barium chloride
Hydrogen can be displaced from a hot alkaline solution by?
- A. Fe
- B. Cu
- C. Ca
- D. Sn
Metals of the first transition series have special properties which are different from those of groups I and II elements because they have partially filled
- A. s orbitals
- B. p orbitals
- C. d orbitals
- D. f orbitals
Chlorine gas is prepared in the laboratory by
- A. adding concentrated hydrochloric acid to solid manganese (IV) oxide
- B. aciding concentrated tetraoxosulphate (VI) acid to solid sodium chloride
- C. dropping concentrated hydrochloric acid onto potassium tetraoxomanganate (VI) crystals
- D. aciding concentrated tetraoxosulphate (VI) acid to hydrochloric acid
Which of the following is an electrolyte?
- A. Alcohol
- B. Sodium acetate solution
- C. Solid potassium hydroxide
- D. Mercury
When sugar is dissolved in tea, the reaction is always accomplished by
- A. positive change in entropy
- B. negative entropy change
- C. no entropy change
- D. a minimum entropy change


