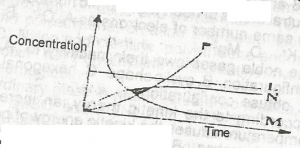
2HCI(ag) + CaCO\(_3\)(s) → CaCl\(_2\)(s) + CO\(_2\)(g) + H\(_2\)O(l). From the reaction above, which of the curves in the diagram represents the production of carbon (IV) oxide as dilute HCI is added?
The correct answer is: B
Explanation
The reaction of calcium carbonate with hydrochloric acid is said to be first order with respect to hydrochloric acid. This is because the rate depends upon the concentration of hydrochloric acid to the power one. As more HCl is being added,more CO\(_2\) is being produced but gradually evolves being a gas, as time goes on. The rate of the reaction is directly proportional to the concentration of a single reactant. This means that if the concentration of the reactant doubles, the rate of the reaction also doubles.


