To obtain pure carbon (II) oxide from its mixture with carbon (IV) oxide, the mixture should be
- A. passed over heated copper (II) oxide
- B. bubbled through water
- C. bubbled through concentrated tetroxosulphate (VI) acid
- D. bubbled through sodium hydroxide
Which of the following alloys contain iron?
- A. Duralumin and steel
- B. Brass and bronze
- C. Steel and permalloy
- D. Soft solder and duralumin
In a flame test for calcium, the flame appears
- A. green when viewed through a blue glass
- B. blue when viewed through a blue glass
- C. orange -red when viewed through a blue glass
- D. brick-red when viewed through a blue-glass
The property used in the industrial preparation of nitrogen and oxygen from air is?
- A. rate of diffusion
- B. density
- C. boiling point
- D. solubility
The ores that can be concentrated by flotation are
- A. nitride ores
- B. sulphide ores
- C. oxide ores
- D. non-metals
Metalliods are also referred to as
- A. semi-metals
- B. metals
- C. colliods
- D. non-metals
The most abundant element on the earth’s crust is
- A. nitrogen
- B. hydrogen
- C. oxygen
- D. flourine
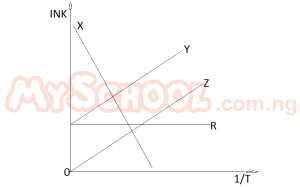
In the diagram above which of the cures illustrates Arrhenius’ law?
- A. Y
- B. Z
- C. R
- D. X
The most suitable metal that can be used as lightning conductor is?
- A. silver
- B. copper
- C. iron
- D. aluminium
NH4Cl(s) ↔ NH3(g) + HCL(g)
The reaction above can only attain equilibrium if
- A. a gaseous reactant is added
- B. one of the products is removed
- C. it is in a closed system
- D. it is an open system
The gas that can be dried using concentrated tetraoxosulphate (VI) acid is
- A. hydrogen bromide
- B. Sulphur (IV) oxide
- C. hydrogen sulphide
- D. ammonia
N2(g) + 3H2(g) ↔ 2NH3(g) ΔH = -90 kJ
In the equation above, the yield of ammonia can be decreased by
- A. increasing the pressure
- B. removing the ammonia as it is formed
- C. increase the temperature
- D. adding a catalyst
A dense white fume is formed when ammonia gas reacts with
- A. O2(g)
- B. H2(g)
- C. Cl2(g)
- D. HCl(g)
When pure aluminium metal is heated to red hot in the presence of nitrogen gas, the compound formed is
- A. Al2N
- B. Al2N2
- C. AlN
- D. Al2N3
The density of a certain gas is 1.98gdm-3 at s.t.p. What is the molecular mas of the gas?
- A. 44.0 g
- B. 54.0 g
- C. 26.0 g
- D. 31.0 g
What is the valence shell electron configuration of the element with atomic number 17?
- A. 1s2 2s2 2p6 3s2 3p4
- B. 1s2 2s2 2p6 3s2 3p5
- C. 2s2 2p6
- D. 3s2 3p5
I. Treatment of cancer
II. Detection of leakages in water mains
III. Detection of the ages of ancient tools
IV. Preparation of drugs
Which combination of the above refers to the uses of radioactive isotopes?
- A. I and III
- B. I and II
- C. I, II and III
- D. I, II, III and IV
The pressure of 100cm3 of oxygen at 35oC is 750mmHg. What will be the volume of gas if the pressure is reduced to 100mmHg without changing the temperature?
- A. 650 cm3
- B. 850 cm3
- C. 580 cm3
- D. 750 cm3
A heterogenous mixture can be defined as any mixture
- A. of a solute nd a solvent
- B. whose cmposition is uniform
- C. whose composition is not uniform
- D. formed by solids and liquids
An element wil readily form an electrovalent compound if it electron configuration is
- A. 2, 8, 1
- B. 2, 8, 4
- C. 2, 8, 8
- D. 2, 8, 5
The component of an atom that contributes least to its mass is the
- A. proton
- B. nucleus
- C. neutron
- D. electron


