(a)(i) Draw and label a diagram to illustrate the preparation and collection of dry chlorine gas in the laboratory.
(ii) List two uses of chlorine.
(b)(i) Explain why river water flowing through an industrial town may be unsafe for drinking.
(ii) State the use of each of the following substances in water treatment: I. Sand, II. Chlorine, III. Calcium oxide, IV. Alum
(c)Consider the reaction represented by the following equation:
2Na\(_2\)CI\(_{(s)}\) H2SO4(aq) \(\to\) Na\(_2\)SO\(_{(4(aq)}\) + 2HCI\(_{(g)}\)
Calculate the volume of HCI gas that can be obtained at s.t.p. from 5.85 g of sodium chloride. [H = 1, Na = 23, CI = 35.5, Molar volume a 22.4 dm\(^3\) at s.t.p]
(d) Give one example in each case of a (i) metal that is a liquid at room temperature. (ii) non-metal that is a iiquid at room temperature, (iii) gas at room temperature that is monatomic.
(e) State two differences between metals and nom metals with respect to their: (i) physical properties; (ii) chemical properties.
(a) A solution of CuSO\(_4\) was electrolyzed between pure copper electrodes and the following results were obtained:
Mass of copper anode before experiment = 7.20 g
Mass of copper anode after experiment = 4.00 g
Mass of copper cathode before experiment = 5.75 g
From the information provided,
(i) calculate the mass of the cathode, after the experiment.
(ii) write an equation for the reaction at the I. anode, II. cathode.
(iii) state whether the colour of the solution would change during the electrolysis. Give a reason for your answer.
(iv) if the electrolysis was carried out for 1 hour 20 minutes with a current of 2.0 amperes, determine the value of the Faraday.
(b) Consider the reaction represented by the following equation:
MnO\(^-_4\) + I\(^-\) + H\(^+\) \(\to\) I\(_2\) + H\(_2\)O + Mn\(^{2+}\)
Write balanced half equation for the (i) oxidation reaction, (ii) reduction reaction.
(c)(i) Describe briefly how tin can be extracted from its ore.
(ii) State one use of tin.
(iii) Mention one property that makes tin suitable for the use stated in (c)(ii)
(d)(i) What is meant by the term pollution?
(ii) Explain why it is dangerous to run a generator in a closed room.
(a) State the following laws of chemical combination: (i) Law of constant composition (ii) Law of multiple’ proportion.
(b) Copper reacts with oxygen to form two oxides X and Y. On analysis, 1.535 g of X yielded 1.365 g copper and 1.450 g of Y yielded 1.160 g of cooper.
i) Determine the chemical formula of X and Y.
(ii) Calculate the mass of copper which can react with 0.500 g of oxygen to yield I. X II. Y.
(iii) Which of the laws of chemical combination is illustrated by the result in (b)(i) above. [ = 16, Cu = 63.51
(c) Write the structure of the product responsible for the observation in each of the following reactions:
(i) A mixture of butanoic acid and ethanol warmed in the presence of concentrated H\(_2\)SO\(_4\) gives off a fragrant odour.
(ii) Sodium dissolves in propan-2-ol with effervescence to give a solution which on evaporation to dryness leaves a white precipitate.
(d) Consider the compound CH\(_3\)CH\(_2\)COOCH\(_2\)CH\(_3\).
(i) Name the compound (ii) Write the structural formula of the compound (iii) State the reagents and conditions for the formation of the compound.
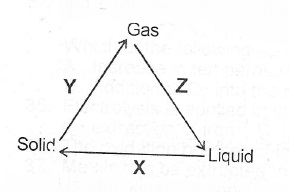
(a)(i) State two differences betwecii the properties of solids and gases
(ii) What process does each of X, Y and Z represent in the changes shown below?
(b)(i) State Charles’ Law (ii) Draw a sketch to graphically illustrate Charles’ Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory.
(ii) Consider the reaction represented by the Solid of Liquid following equation:
H\(_{2(g)}\) + Cl\(_{2(g)}\) \(\to\) 2HCI\(_{(g)}\)
Use the kinetic theory to explain how the rate of formation of HCI\(_{(g)}\) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case vihich produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.
a) Define each of the following terms and indicate one use of each:
(i) Nuclear fission; (ii) Nuclear fusion.
(b) Alpha particle emission by \^293_25U\) proceduces an element A. Beta particle emission by the particle A produces another element B. Element B also undergoes alpha particle emission to produce \(^227_89AC\). Write balanced equations to represent the above statement.
(c) The models below represent the filling of orbitals in an atom.
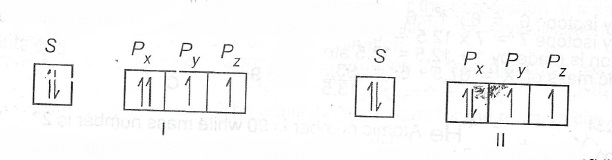
State which rule(s) is/are violated or obeyed by each model.
(d) Explain why the boiling point of H\(_2\)S with relative molecular mass of 34 is lower than that of H\(_2\)O with relative molecular mass of 18.
(e) HCI is passed into each of the following solvents:
(i) water;
(ii) methylbenzene. I. State the effect of each solution on blue litmus paper II. Compare the electrical conductivities of the two solutions.
(f) Zinc dust is added to copper (II) tetraoxosulphate (VI) solution. State;
(i) what is observed; (ii) the type of reaction that occurs.
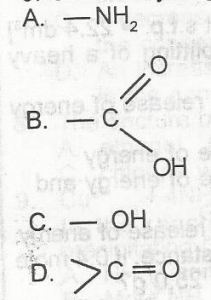
Which of the following functional groups will give gas bubbles when treated with a saturated solution of sodium hydrogen trioxocarbonate (IV)?
- A. A
- B. B
- C. C
- D. D
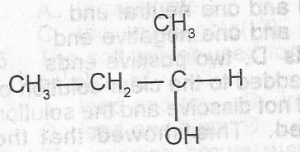
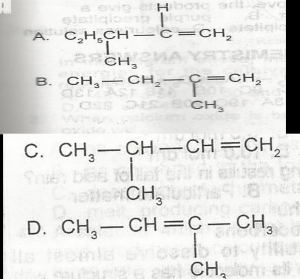
The oxidation of the compound above produces
- A. 2-butanone
- B. 2-butanal
- C. 3-butanal
- D. 3-butanone
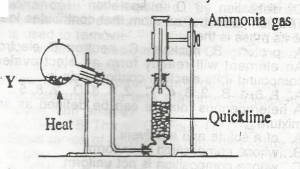
In the diagram above, mixture Y is
- A. NH4NO3(s) + CaSO4(s),/sub>
- B. NH4CL(s) + NaNO2(s)
- C. NH4NO2(s) + Na2SO4(s)
- D. NH4CL(s) + Ca(OH)2(s)
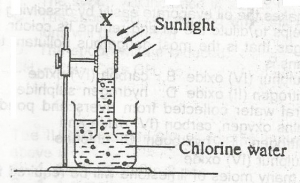
In the diagram above, gas X is
- A. hydrogen
- B. chlorine
- C. oxygen
- D. hydrogen chloride
5SO2(g) + 2KMnO4(aq) + 2H2O(l) → K2SO4 + 2MnSO4(aq) + 2H2SO4(aq)
In the reaction above, the products give a
- A. purple solution
- B. purple precipitate
- C. colourless precipitate
- D. colourless solution
If the heat of combustion of hydrogen is -285.8kJ,
What is the heat of formation of water?
- A. +571.6 kJ
- B. -571.6 kJ
- C. -285.8 kJ
- D. +285.8 kJ
Na2CO3(g) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
How many moles of sodium trioxocarbonate (IV) are there in a 25 cm3 solution which required 10cm3 of 0.05 mol dm-3 hydrochloric acid solution to neutralize it?
- A. 1.000 mole
- B. 0.100 mole
- C. 0.010 mole`
- D. 0.111 mole
In the electrolysis of CuSO4(g) using platinum electrodes, the reaction at the anode is
- A. 4H+ + 4e- → 2H2
- B. 4OH- + 4e- → 2H2O + O2
- C. 2OH - 2e- → 2OH
- D. 2OH- + 2OH- → 2H2O + O2
In which of the following is the oxidation number of sulphur equal to -2?
- A. SO3
- B. S8
- C. H2S
- D. SO2
The solution that will conduct the highest amount of electricity is?
- A. 0.5 mol dm-3 of hydrochloric acid
- B. 2.0 mol dm-3 of hydrochloric acid
- C. 0.5 mol dm-3 ethanoic acid
- D. 2.0 mol dm-3 ethanoic acid
Zinc and carbon rods are used as anode and cathode respectively in a
- A. Leclanche cell
- B. lead accumulator
- C. Daniel cell
- D. voltaic cell
H2(g) + Br2(g) → 2HBr(g)
The above reaction is carried out at 25oC. ΔH is -72 kJ mol-1 and ΔS is – 106 J mol-1K-1, the reaction will?
- A. not proceed spontaneously at the given
- B. proceed spontaneously at the given temperature
- C. proceed in the reverse direction at the given temperature
- D. proceed spontenously at lower temperature
The ion that can be used as an oxidizing agent is
- A. Au3+
- B. Fe3+
- C. ClO-
- D. Ca2+
2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g)
In the reaction above, the mass of carbon(IV)oxide produced on burning 78 g of ethyne is
[C =12, O = 16, H = 1]
- A. 264 g
- B. 39 g
- C. 352 g
- D. 156 g
If 30cm3 of gas at 50oC is warmed to 80o at a fixed pressure, the fractional increase in volume is
- A. 0.009
- B. 0.093
- C. 0.910
- D. 1.090