(a)(i) State two differences betwecii the properties of solids and gases
(ii) What process does each of X, Y and Z represent in the changes shown below?
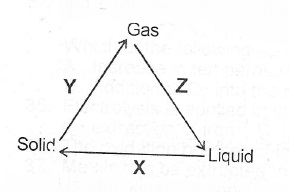
(b)(i) State Charles’ Law (ii) Draw a sketch to graphically illustrate Charles’ Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory.
(ii) Consider the reaction represented by the Solid of Liquid following equation:
H\(_{2(g)}\) + Cl\(_{2(g)}\) \(\to\) 2HCI\(_{(g)}\)
Use the kinetic theory to explain how the rate of formation of HCI\(_{(g)}\) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case vihich produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.

