If the electron configuration of an element is 1S\(^2\) 2S\(^2\)2P\(^5\), how many unpaired electron are there?
The correct answer is: C
Explanation
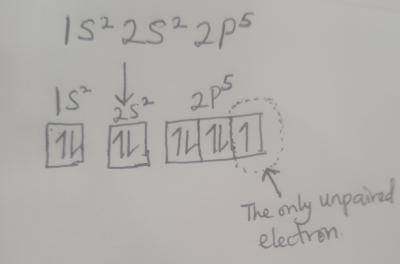
When the electrons are drawn out in the orbitals, following the Hund's rule, the diagram above is what will be obtained.


