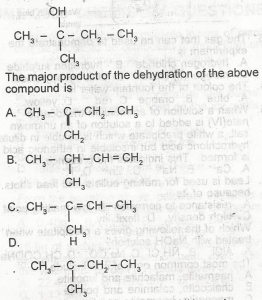
Choose the correct option from the structure above
- A. A
- B. B
- C. C
- D. D

Use the diagram to answer this question.The colour of the fountain water is
- A. blue
- B. orange
- C. red
- D. yellow
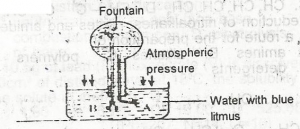
Use the diagram to answer this question. The gas that can be used to demonstrate the experiment is
- A. hydrogen chloride
- B. hydrogen sulphide
- C. nitrogen(ll) oxide
- D. dinitrogen (l) oxide
Detergents are manufactured with straight hydrocarbon chains so as to make them
- A. soluble
- B. biodegradable
- C. cheaper
- D. foamy
Polyvinyl chloride is used in the production of
- A. glass
- B. alloy
- C. pipes
- D. ceramics
The product obtained when a mixture of benzene vapour and hydrogen are passed over nickel catalyst at 180ºC is
- A. cyclohexane
- B. cyclopentane
- C. n- hexane
- D. n - pentane
Reduction of alkanones with LialH4 produces
- A. primary alkanols
- B. secondary alkanols
- C. tertiary alkanols
- D. polyhydric alkanols
Reduction of nitroalkanes, nitrites and amides is a route for the preparation of
- A. amines
- B. alkenes
- C. polymers
- D. detergents
A red precipitate of copper (I) carbide is formed when ammonium solution of copper (I) chloride is introduced into
- A. CH2 = CH - CH2 - CH3
- B. CH3 CH2 - C ≡ CH
- C. CH3 CH2 CH2 CH3
- D. CH3 - C C - CH3
A hydrocarbon X with a molar mass of 26 consists of 92.3% carbon. What is its molecular formular?
- A. C2H2
- B. C3H3
- C. C4H4
- D. C5H5
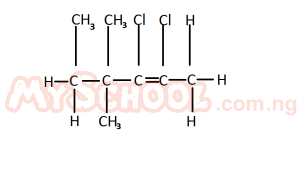
The IUPAC nomenclature for the structure above is
- A. 2, 3 - dichloro - 4, 4, 5 - trimethyl pent - 2 - ene
- B. 4, 5 - dichloro - 2, 3 - dimethyl hex - 2 - ene
- C. 2, 3 - dichloro - 4, 4 - dimethyl hex - 2- ene
- D. 2, 3 dichloro - 2, 2 - dimethyl hex - 2 - ene
If glucose is heated with concentration tetraoxosulphate (VI) acid, it will be dehydrated to
- A. carbon
- B. carbon (IV) oxide
- C. ethene
- D. ethanol
If the silver mirror test is positive, it indicates the presence of an
- A. alkyne
- B. alkanol
- C. alkanone
- D. alkanal
The type of isomerism shown by cis- and trans- isomers is
- A. optical isomerism
- B. positional isomerism
- C. functional isomerism
- D. geometrical isomerism
The most common ores of iron include
- A. haematite, malachite and limonite
- B. chalcocite, calamine and bornite
- C. magnetite, haematite and limonite
- D. magnetite, chalcocite and bornite
which of the following gives a precipitate when treated with NaOH solution?
- A. AlCl3
- B. NH4Cl
- C. Na2CO3
- D. CH3COONa
Leads is used for making bullets and lead shots because of its
- A. resistance to corrosion
- B. low melting point
- C. high density
- D. flexibility
When a solution of ammonium trioxocarbonate (IV) is added to a solution of an unknown salt, a white precipitate which is soluble in dilute hydrochloric acid but insoluble in ethanoic acid is formed. This indicates the presence of
- A. Ca2+
- B. Na+
- C. Zn2+
- D. K+
Which of the following compounds of trioxonitrate (V) will decompose to give dinitrogen (i) oxide and water when heated?
- A. NaNO3
- B. Zn(NO3)2
- C. Cu(NO3)2
- D. NH4NO3
The substance that is used in the steel industry for the removal of carbon, sulphur and phosphorus impurities from pig iron is
- A. oxygen
- B. chlorine
- C. nitrogen
- D. hydrogen
The rate of a reaction usually decreases with a decrease in the concentration of reactants because
- A. kinetic energy decrease
- B. temperature increase
- C. speed increases
- D. reactants collision decrease


