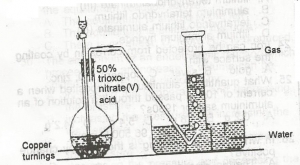
In the diagram above, the gas produced is?
- A. NO
- B. NO2
- C. N2O
- D. N2O4
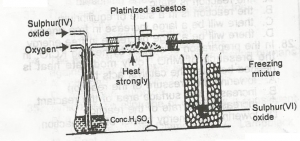
In the diagram above, the purpose of the asbestos to?
- A. absorb impurities
- B. catalyze the reaction
- C. solidify the gas
- D. dry the gas
The compound that is used as an anesthetic is?
- A. CCl4
- B. CH Cl3
- C. CH2Cl2
- D. CH3Cl
Two hydrocarbons X and Y were treated with bromine water. X decolorised the solution and Y did not not. Which class of compound does Y belong?
- A. Benzene
- B. Alkynes
- C. Alkenes
- D. Alkanes
The haloalkanes used in dry-cleaning industries are?
- A. trichloromethane and tetrachloromethane
- B. chloroethene and dichloroethene
- C. trichloroethene and tetrachloroethene
- D. chloroethane and dichloroethene
One of the major uses of alkane is?
- A. as domestic and industrial fuel
- B. in the hydrogenation of oils
- C. in the textile industries
- D. in the production of plastics
The correct order of increasing boiling points of the following compounds C3H7OH, C7H16 and C4H10 is?
- A. C3H7OH → C4H10 → C7H10
- B. C4H10 → C7H16 → C3H7OH
- C. C7H16 → C3H7OH → C4H10
- D. C4H10 → C3H7OH → C7H16
The final products of the reaction between methane and chlorine in the presence of ultraviolet light are hydrogen chloride and?
- A. tricloromethane
- B. dichloromethane
- C. tetrachloromethane
- D. chloromethane
Which of the following is used to hasten the ripening of fruit?
- A. Ethene
- B. Ethanol
- C. Ethyne
- D. Ethane
Benzene reacts with hydrogen in the presence of nickel catalyst at 180ºC to give?
- A. xylene
- B. toluene
- C. cyclopentane
- D. cyclohexane
Which of the following organic compounds is very soluble in water?
- A. CH3COOH
- B. C2H2
- C. C2H4
- D. CH3COOC2H5
Which of the following is used as fuel in miners’ lamp?
- A. Ethanal
- B. Ethyne
- C. Ethene
- D. Ethane
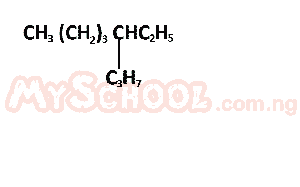
The IUPAC nomenclature of the compound above is?
- A. 4 - ethyloctane
- B. 5 - ethyloctane
- C. 5 - propylheptane
- D. 3 - propylheptane
The residual solids from the fractional distillation of petroleum are used as?
- A. coatings of pipes
- B. raw materials for the cracking process
- C. fuel for the driving tractors
- D. fuel for jet engines
Stainless steel is used for making?
- A. magnets
- B. tools
- C. coins and medals
- D. moving parts of clocks
A compound gives an orange-red color to non-luminous flame. This compound is likely to contain?
- A. Na+
- B. Ca2+
- C. Fe3+
- D. Fe2+
When iron is exposed to moist air, it gradually rusts. This is due to the formation of?
- A. hydrate iron (III) oxide
- B. anhydrous iron (III) oxide
- C. anhydrous iron (II) oxide
- D. hydrate iron (II) oxide
A constituent common to bronze and solder is?
- A. lead
- B. silver
- C. copper
- D. tin
Which of the following is used in rocket fuels?
- A. HNO3
- B. CH3COOH
- C. H2SO4
- D. HCl
To a solution of an unknown compound, a little dilute tetraoxosulphate (VI) acid was added with some freshly prepared iron (II) tetraoxosulphate (VI) solution. The brown ring observed after the addition of a stream of concentrated tetraoxosulphate (VI) acid confirmed the presence of?
- A. CO\(^{2-}_{3}\)
- B. Cl-
- C. SO\(_{3}^{2-}\)
- D. NO\(^{2-}_{3}\)
2H2(g) + O2(g) ⇌ 2H2O(g) ΔH = -ve
what happens to the equilibrium constant of the reaction above if the temperature is increased?
- A. it is unaffected
- B. it becomes zero
- C. it decrease
- D. it increase


