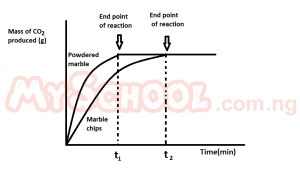
The graph above demonstrate the effect of?
- A. surface area on the rate of reaction
- B. catalyst on the rate of reaction
- C. pressure on the rate reaction
- D. concentration on the rate of reaction
In the preparation of oxygen by heating KClO3 in the presence of MnO2, only moderate heat is needed because the catalyst acts by?
- A. lowering the pressure of the reaction
- B. increasing the surface area of the reactant
- C. increase the rate of the reaction
- D. lowering the energy barrier of the reaction
If a reaction is exothermic and there is a great disorder, it means that?
- A. the reaction is static
- B. the reaction is in a state of equilibrium
- C. there will be a large increase in free energy
- D. there will be a large decrease in free energy
In which of the following is the entropy change positive?
- A. Thermal dissociation of ammonium chloride
- B. Reaction between an acid and a base
- C. Addition of concentrated acid to water
- D. Dissolution of sodium metal in water
What quantity of aluminum is deposited when a current of 10A is passed through a solution of an aluminum salt for 1930s?
[Al = 27, F = 96500 C mol -1]
- A. 0.2 g
- B. 1.8 g
- C. 5.4 g
- D. 14.2 g
Iron can be protected from corrosion by coating the surface with?
- A. gold
- B. silver
- C. copper
- D. zinc
The IUPAC nomenclature of the compound LiAlH4 is?
- A. lithiumtetrahydridoaluminate (III)
- B. aluminium tetrahydrido lithium
- C. tetrahydrido lithium aluminate (III)
- D. lithium aluminium hydride
6\(AgNO_{4(aq)} + PH_{3(g)} + 3H_2O_{(l)}\) → 6\(Ag_{(s)} + H_3PO_{3(g)} + 6HNO_{3(aq)}\)
In the above reaction, the reducing agent is?
- A. HNO3(aq)
- B. H2O(l)
- C. PH3(g)
- D. AgNO3(aq)
Which of the following salts is slightly soluble in water?
- A. AgCl
- B. CaSO4
- C. Na2CO3
- D. PbCl2
The colour of methyl orange in alkaline medium is?
- A. yellow
- B. pink
- C. orange
- D. red
Calculate the volume of 0.5 mol dm-3 H2So4 that is neutralized by 25 cm3 of 0.1 mol dm-3 NaOH?
- A. 5.0 cm3
- B. 2.5 cm3
- C. 0.4 cm3
- D. 0.1 cm3
The acid that is used to remove rust is?
- A. boric
- B. hydrochloric
- C. trioxonitrate (V)
- D. tetraoxosulphate (VI)
Carbon (II) oxide is considered dangerous if inhaled mainly because it?
- A. can cause injury to the nervous system
- B. competes with oxygen in the blood
- C. competes with carbon (IV) oxide in the blood
- D. can cause lung cancer
Coffee stains can best be removed by?
- A. Kerosine
- B. turpentine
- C. a solution of borax in water
- D. ammonia solution
Calculate the solubility in mol dm-3 of 40g of CuSO4 dissolved in 100g of water at 120°C?
[Cu = 64, S = 32, O = 16]
- A. 4.00
- B. 2.50
- C. 0.40
- D. 0.25
Substance employed as drying agents are usually?
- A. amphoteric
- B. hygroscopic
- C. efflorescent
- D. acidic
Permanent hardness of water can be removed by?
- A. filtration
- B. adding slaked lime
- C. adding caustic soda
- D. boiling
A noble gas with a high power of fog penetration used in aerodrome beacons is?
- A. krpton
- B. argon
- C. helium
- D. neon
12D + 13T → 24He + 01n + energy
The reaction above illustrates?
- A. alpha decay
- B. artificial transmutation
- C. nuclear fussion
- D. nuclear fission
Elements in the same period in the periodic table have the same?
- A. number of shells
- B. atomic number
- C. chemical properties
- D. physical properties
The maximum number of electrons in the L shell of an atom is?
- A. 2
- B. 8
- C. 18
- D. 32


