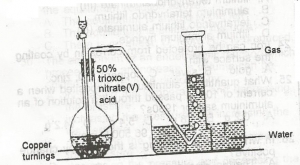
In the diagram above, the gas produced is?
The correct answer is: B
Explanation
In this laboratory preparation, 50% trioxonitrate (V) acid is reacted with copper turnings to liberate nitrogen (II) oxide ( NO ) that is collected by downward delivery.
Cu+\(4HNO_3 →Cu(NO_3)_2+2NO_2+2H_2O\)
There is an explanation video available .


