The principle of column chromatography is based on the ability of the constituents to
- A. move at different speeds in the column
- B. dissolve in each other in the column
- C. react with the solvent in the column
- D. react with each other in the column

choose the correct answer in the option above. Which of the following is a primary amine?
- A. A
- B. B
- C. C
- D. D
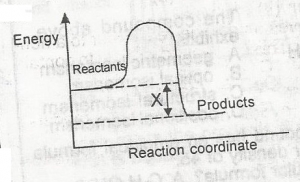
In the diagram above , X is the “
- A. enthalpy
- B. enthalpy change
- C. activation energy
- D. activated complex
with conc H2SO4 as catalyst
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
- A. esterification
- B. Condensation
- C. saponification
- D. neutralization
Which of the following statements is true about 2-methylpropane and butane
- A. They are not members of the same homologous series
- B. They have the same boiling point
- C. They have different number of carbon atoms
- D. They have the same chemical properties
Two organic compounds K and L were treated with a few drops of Fehling’s solutions respectively . K formed a brick-red precipitate while L, remains unaffected. The compound K is an
- A. alkanol
- B. alkane
- C. alkanal
- D. alkanone
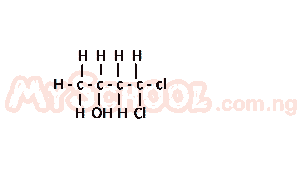
The functional groups present in the compound above are
- A. alkene and halo-group
- B. hydroxyl and chloro-group
- C. alkene and chloro-group
- D. hydroxyl and halo-group
The number of isomers that can be obtained from C4H10 is
- A. 3
- B. 4
- C. 1
- D. 2
At 25o and zymase as catalyst,
C6H126 → 2C2H5OH + 2CO2 + energy
The reaction above represented by the equation above is useful in the production of
- A. propanol
- B. butanol
- C. methanol
- D. ethanol
An organic compound has an empirical formula CH2O and vapour density of 45.
What is the molecular formula?
[C = 12, H = 1, O = 16]
- A. C3H7OH
- B. C2H5OH
- C. C3H6O3
- D. C2H4O2
Which of the following compounds will undergo polymerization reaction?
- A. C2H4
- B. C2H5COOH
- C. C2H6
- D. C2H5OH
The ability of carbon to form long chains is referred to as
- A. alkylation
- B. acylation
- C. catenation
- D. carbonation
The constituents of baking powder that makes the dough to rise is
- A. NaHCO3
- B. NaOH
- C. Na2CO3
- D. NaCl
Bronze is preferred to copper in the making of medals because it
- A. is stronger
- B. can withstand low temperature
- C. is lighter
- D. has low tensile strength
The property of concentrated H2SO4 that makes it suitable for preparing HNO3 is its
- A. boiling point
- B. density
- C. oxidizing properties
- D. dehydrating properties
In the laboratory preparation of oxygen, dried oxygen is usually collected over
- A. hydrochloric acid
- B. mercury
- C. calcium chloride
- D. tetraoxosulphate (VI) acid
The bleaching action of chlorine gas is effective due to the presence of
- A. hydrogen chloride
- B. water
- C. air
- D. oxygen
A few drops of concentrated HNO\(_3\) is added to an unknown solution and boiled for a while. If this produces a brown solution, the cation present is likely to be
- A. Pb2+
- B. Cu2+
- C. Fe3+
- D. Fe2+
The effect of the presence of impurities such as carbon and sulphur on iron is that they
- A. give it high tensile strenght
- B. make it malleable and ductile
- C. increase its melting point
- D. lower its melting point
The impurities formed during the laboratory preparation of chlorine gas are removed by
- A. H2O
- B. NH3
- C. H2SO4
- D. HCl
Given that M is the mass of a substance deposited during electrolysis and Q is the quantity of electricity consumed, then Faraday’s first law can be written as
[Electrochemical equivalent]
- A. M = E/Q
- B. M = EQ
- C. M = Q/E
- D. M = E/2Q


