2SO2(g) + O2(g) \( \iff \)2SO3(g)
\( \bigtriangleup H = -395.7kJmol^{-1} \)
In the reaction above, the concentration of 2SO3(g) can be in creased by
- A. decreasing the pressure
- B. decreasing the temperature
- C. the addition of catalyst
- D. increasing the temperature
Zn(s) + CuSO4(aq) \( \to \) ZnSO4(aq) + Cu(s)
In the reaction above, the oxidizing agent is
- A. CuSO4(aq)
- B. ZnSO4(aq)
- C. Cu(s)
- D. Zn(s)
The radioactive emission with the least ionization power is
- A. \( \alpha - particles \)
- B. X-rays
- C. \( \gamma -rays \)
- D. \( \beta - rays \)
How many unpaired electron are in the p-orbitals of a fluorine atom?
- A. 3
- B. \( 0 \)
- C. 1
- D. 2
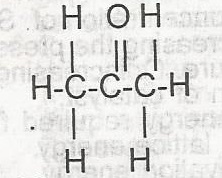
The compound above is an
- A. alkanone
- B. alkanoate
- C. alkanal
- D. akanol
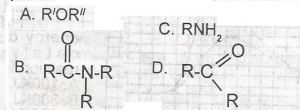
Use the following option above to answer this question. Which of the following compounds in solution will turn red litmus paper blue?
- A. A
- B. B
- C. C
- D. D
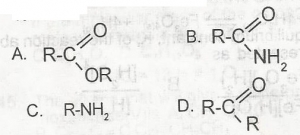
Use the above option to answer this question. The dehydration of ammonium salt of alkanoic acids produces a compound with the general formula
- A. A
- B. B
- C. C
- D. D
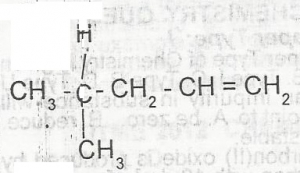
The IUPAC nomenclature for the compound above is
- A. 4-methylpent-1-ene
- B. 3-methylpent-2-ene
- C. 2-methylpent-1ene
- D. 2-methylpent-4-ene

Choose the correct option from the graph above . 3Fe(S) + 4H2O(g) ⇌ Fe3O4(s) + 4H2(g). The equilibrium constant, K, of the reaction above is represented as
- A. A
- B. B
- C. C
- D. D
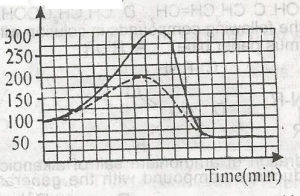
In the graph above, the activation energy of the catalyzed reaction is
- A. 100KJ
- B. 300KJ
- C. 250KJ
- D. 200KJ
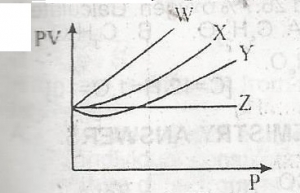
From the diagram above, an ideal gas is represented by
- A. Z
- B. W
- C. X
- D. Y
An organic compound contains 60% carbon, 13.3% hydrogen and 26.7% oxygen. Calculate the empirical formula
rn(C=12,H =1, O=16)
- A. C 5H 12O
- B. C 3H 8O
- C. C 6H 13O 2
- D. C 4H 9O
Which of the following fraction is used as raw material for the cracking process?
- A. kerosine
- B. lubricating oil
- C. bitumen
- D. diesel oils
A primary amide is generally represented by the formula
- A. RCOOR
- B. RCONH 2
- C. RCONHR
- D. RCONR 2
The decarboxylation of ethanoic acid will produce carbon (IV)oxide and
- A. methane
- B. ethane
- C. propane
- D. butane
The reaction between ammonia and ethyl ethanoate produces
- A. propanol and ethanamide
- B. propanol and propanamide
- C. ethanol and propanamide
- D. ethanol and ethanamide
2- methylpropan-2-ol is an example of a
- A. dihydric alkanol
- B. tertiary alkanol
- C. secondary alkanol
- D. primary alkanol
Aluminium containers are frequently used to transport trioxonitrate (V) acid because aluminium
- A. has a silvery-white appearance
- B. has a low density
- C. does not react with the acid
- D. does not corrode
The coloured nature of transition metal ions are associated with their partially filled
- A. f- orbital
- B. s- orbital
- C. p-orbital
- D. d-orbital
Copper is displaced from the solution of its salts by most metals because it
- A. is a transition element
- B. is at the bottom of the activity series
- C. is very reactive
- D. has completely filled 3d-orbitals
A metal that forms soluble trioxosulphate (IV) ion is
- A. barium
- B. potassium
- C. manganese
- D. aluminium


