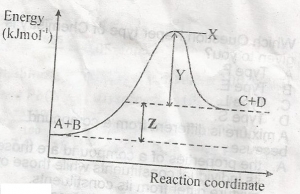
Z in diagram above represents
- A. heat of reaction
- B. activation energy
- C. free energy
- D. entropy of reaction

In the diagram, the function of the concentrated H2SO4 is to?
- A. purify the gas
- B. dry the gas
- C. liquefty the gas
- D. remove odour
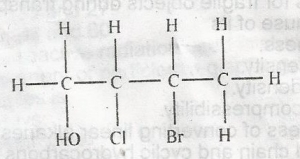
The IUPAC nomenclature of the compound above is
- A. 2-bromo-3-chlorobutanol
- B. 3-bromo-2-chlorobutanol
- C. 3-chloro-2-bromobutanol
- D. 2-chloro-3-bromobutanol
The petroleum fraction that is used in heating furnaces in industries is
- A. diesel oil
- B. gasoline
- C. kerosene
- D. lubricating oil
The process of converting linear alkanes to branched chain and cyclic hydrocarbons by heating in the presence of a catalyst to improve the quality of petrol is referred to as
- A. refining
- B. cracking
- C. reforming
- D. blending
Polystyrene is widely used as packaging materials for fragile objects during transportation because of its
- A. lightness
- B. low density
- C. high density
- D. high compressibility
A hydrocarbon has an empirical formula CH and a vapour density of 39. Determine its molecular formula.
[C = 12, H = 1]
- A. C2H6
- B. C3H8
- C. C3H4
- D. C6H6
CH3COOC2H5(1) + H2O(1) ⇌ CH 3COOH (aq) + C 2H 5OH (aq)
The purpose of H+ in the reaction above is to
- A. increase the yield of products
- B. maintain the solution at a constant PH
- C. increase the rate of the hydrolysis
- D. decrease the rate of the reverse reaction
The polymer used in making car rear lights is
- A. perspex
- B. bakeelite
- C. polystyrene
- D. polyacrylonitrile
The process of converting starch to ethanol is
- A. cracking
- B. distillation
- C. fermentation
- D. oxidation
Due to the unstable nature of ethyne, it is stored by dissolving in
- A. ethane-1,2-diol
- B. propanol
- C. ethanoic acid
- D. propanone
Ethyne is passed through a hot tube containing organo-nickel catalyst to produce
- A. isoprene
- B. polythene
- C. ethanol
- D. benzene
The alkanol obtained from the production of soap is
- A. propanol
- B. ethanol
- C. glycerol
- D. methanol
The general formula of haloalkanes where X represents the halide is
- A. Cn H 2n-1X.
- B. C nH 2nX.
- C. C nH 2n+2 X
- D. C nH 2n+1X
A few drops of NaOH solution was added to an unknown salt forming a white precipitate which is insoluble in excess solution. The cation likely present is
- A. Zn2+
- B. Pb2+
- C. Ca2+
- D. Al3+
A compound that gives a brick-red colour to a non-luminous flame is likely to contain
- A. copper ions
- B. sodium ions
- C. calcium ions
- D. Potassium ions
In the electrolytic extraction of calcium from calcium chloride, the cathode is
- A. zinc
- B. graphite
- C. platinum
- D. iron
In the extraction of sodium from fused sodium chloride, the anode is made of platinum because
- A. sodium is formed at the anode
- B. chlorine is formed at the anode
- C. sodium does not react with platinum
- D. chlorine does not react with platinum
Fluorine does not occur in the free state in nature because
- A. it is a poisonous gas
- B. it belongs to the halogen family
- C. it is inert
- D. of its high reactivity
In a chemical reaction, the change in concentration of a reactant with time is
- A. entropy of reaction
- B. enthalpy of reaction
- C. rate of reaction
- D. order of reaction
In an equilibrium reaction, which of the following conditions indicates that maximum yield of the product will be obtained?
- A. Equilibrium constant is very large
- B. ∆H - T∆S = O
- C. ∆ H > T ∆ S
- D. Equilibrium constant is less than zero


