P1V1 = P2V2 supports ?
- A. Charles’s law
- B. Boyles’s law
- C. Graham’s law
- D. Avogadro’s law
Tartaric acid is used industrially to
- A. make baking powder
- B. make fruit juice
- C. remove rust
- D. dry substance
A liquid that will dissolve fat is
- A. hydrochloric acid
- B. calcium hydrochloride
- C. kerosene
- D. water
In the production of soap, concentrated sodium chloride solution is added to
- A. increase the solubility of soap
- B. decrease the solubility of the soap
- C. saponify the soap
- D. emulsify the soap
Which of the following metals burns with brick red
- A. Pb
- B. Ca
- C. Na
- D. Mg
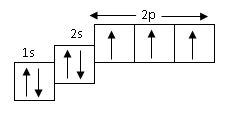
The above orbital diagram shown the electronic configuration of
- A. chlorine
- B. nitrogen
- C. calcium
- D. neon
A suitable reagent for distinguish between ethanoic and ethanol is
- A. bromine water
- B. Fehling’s solution
- C. sodium hydrogen trioxocarbonate (iv)
- D. Ammoniacal silver trioxonitrate(V)
Flow of current in electrolytes is due to the movement of
- A. electrons
- B. Holes and electron
- C. Ions
- D. Charges
Cathode rays cause an object placed behind a perforated anode to cast a shadow on the screen. This observation shows that the rays
- A. are positively charged
- B. are negatively charged
- C. Have mass
- D. travel in straight lines
An elements used in production of matches is
- A. nitrogen
- B. aluminum
- C. copper
- D. sulphur
The weakest attractive force that can be observed between two molecules is
- A. ionic
- B. covalent
- C. co-ordinate covalent
- D. vander Waals
Which of the noble gases has the greatest ionization energy
- A. He
- B. Xe
- C. Ar
- D. Rr
When salt loses its water of crystallization to the atmosphere on exposure, the process is said to be
- A. efflorescence
- B. déliquescence
- C. effervescence
- D. fluorescence
A small quantity of solid ammonium chloride (NH4Cl) heated gently in a test tube, the solid gradually disappear produce two gases. Later, a white cloudy deposit was absence on the cooler part of the test tube. The ammonium chloride is to have undergo
- A. distillation
- B. sublimation
- C. precipitation
- D. evaporation
The acid in electrolysis of water is dilute
- A. HNO3
- B. CH3COOH
- C. H2SO4
- D. HCl
Vulcanization involve the removal of
- A. monometer
- B. the single bond
- C. the double bond
- D. a polymer
The gas that is most useful in protecting humans against solar marathon is
- A. chlorine
- B. ozone
- C. carbon IV oxide
- D. hydrogen sulphide
In the industrial production of H2 from water gas the CO2 produced along with the H2 is removed by
- A. washing under pressure
- B. drying over phosphorus (V) oxide
- C. passing the mixture to the limewater
- D. using ammonical copper (I) chlorine
Iron is often galvanized in order to
- A. Make it more malleable
- B. Remove the impurities unit
- C. Protect it against corrosion
- D. Render it passive
Which of these require crystallization most?
- A. Drug making
- B. Cement making
- C. Paint making
- D. Perfume making


