N2O4(aq) \(\rightleftharpoons\) 2NO2(g) \(\bigtriangleup\)H = +ve
In the reaction above, an increase in temperature will
- A. Increase the reactant production
- B. Increase the value of the equilibrium constant
- C. Shift the equilibrium to the left
- D. Decrease the value of the equilibrium constant
CH4(g) + CI2(g) \(\to\) CH2CI(s) + HCIg
The major factor that influences the rate of the reaction above is
- A. Concentration
- B. Catalyst
- C. Temperature
- D. Light
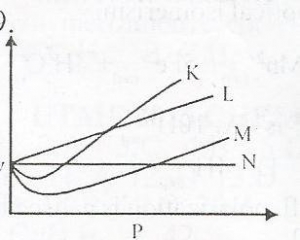
From the diagram above, an ideal gas can be represented by
- A. K
- B. M
- C. L
- D. N
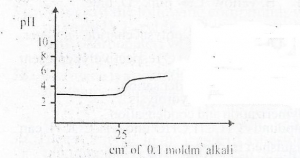
The curve depicts titration between a strong acid of pH
- A. Strong acid and strong base
- B. Strong acid and weak base
- C. Weak acid and weak base
- D. Weak acid and strong base
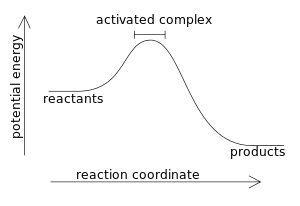
The diagram represents
- A. A spontaneous reaction
- B. An exothermic reaction
- C. A non-spontaneous
- D. An endothermic reaction
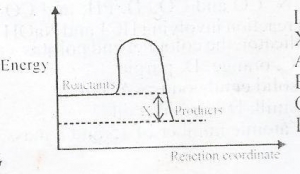
In the diagram above, X is the
- A. Enthalphy
- B. Activated complex
- C. Activation energy
- D. Enthalphy change
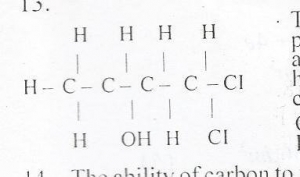
The functional groups present in the compound above are
- A. Alkene and halo-group
- B. Hydroxyl and chloro-group
- C. Alkene and chloro-group
- D. Hydroxyl and halo-group
n monosaccharide \( \underset{Q}{\stackrel{P}{\rightleftharpoons}} \) polysaccharide –n water.
In the process above, P and Q respectively represent
- A. Condensation and hydrolysis
- B. Fermentation and condensation
- C. Polymerization and hydrolysis
- D. Polymerization and condensation
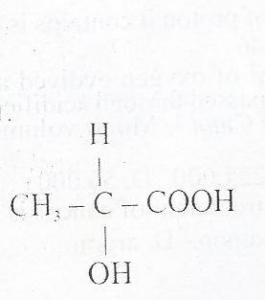
The compound above exhibits
- A. Geometric isomerism
- B. Positional isomerism
- C. Structural isomerism
- D. Optical isomerism
MnO\(_4\)(aq) + Y + 5Fe\(^{2+}\) (aq) \(\mapsto\) Mn\(^{2+}\) (aq) + 5Fe\(^{3+}\) (aq) + 4H\(_2\)O (l).
In the equation above, Y is
- A. 10H+ (aq)
- B. 5H+ (aq)
- C. 8H+ (aq)
- D. 4H+ (aq)
A chemical reaction in which the hydration energy is greater than the lattice energy is referred to as
- A. A reversible reaction
- B. A spontenous reaction
- C. An endothermic reaction
- D. An exothermic reaction
The gas that is used for the treatment of cancer is
- A. Neon
- B. Radon
- C. Xenon
- D. Argon
Calculate the volume in cm3 of oxygen evolved at s.t.p when a current of 5A is passed through acidified water for 193s?
[F = 96500 C mol1. Molar volume of a gas at s.t.p = 22.4dm3]
- A. 0.056dm3
- B. 0.224dm3
- C. 224.000dm3
- D. 56.000dm3
An isotope has an atomic number of 15 and a mass number of 31. The number of proton it contains is
- A. 16
- B. 15
- C. 31
- D. 46
An example of a solid emulsion is
- A. Butter
- B. Hair cream
- C. Milk
- D. Cod-liver oil
In a neutralization reaction involving HCI and NaOH using litmus as indicator, the colour at the end point is
- A. Blue
- B. Red
- C. Orange
- D. Purple
In the extraction of iron, the waste gas from the furnace is a mixture of
- A. PH3 CO and CO2
- B. CO and C
- C. N2 , CO and CO2
- D. PH3 and CO
The arrangement of particles in crystal lattices can be studied using
- A. X-rays
- B. \(\beta\)-rays
- C. \(\alpha\)-rays
- D. \(\gamma\)-rays
The drying agent suitable for drying ammonia is
- A. Calcium Chloride
- B. Calcium Oxide
- C. Phosphorus(V) Oxide
- D. Concentrated H2SO4
The number of electronic shells contained in an atom with electron configuration 1s22s22p63s23p64s2 is
- A. 3
- B. 2
- C. 4
- D. 5
Ethanol is soluble in water due to the presence of a
- A. Carbonyl group
- B. Hydroxyl group
- C. Phenyl group
- D. Methyl group


