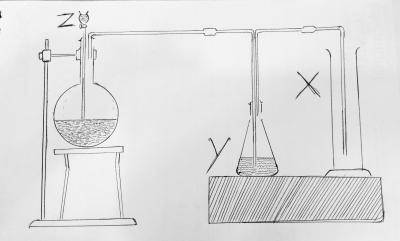
X(g) + 3Y(g) —- 2z(g) H = +ve. if the reaction above takes place at room temperature, the G will be
- A. negative
- B. zero
- C. positive
- D. indeterminate
Ca(OH)2(s) + 2NH4Cl(s) → CaCl2(s) + 2H2O(l) + X. In the reaction above X is
- A. NO2
- B. NH3
- C. N2O
- D. NO2
Water for town supply is chlorinate to make it free from
- A. bad colour
- B. bacteria
- C. temporary hardness
- D. permanent hardness
The furring of kettles is caused by the presence in water of
- A. calcium hydrogentrioxocarbonate (IV)
- B. calcium trioxocarbonate (IV)
- C. calcium tetraoxosulphate (VI)
- D. calcium hydroxide
Due to the high reactivity of sodium, it is usually stored under
- A. water
- B. mercury
- C. paraffin
- D. phenol
If 100cm3 of oxygen pass through a porous plug is 50 seconds, the time taken for the same volume of hydrogen to pass through the same porous plug is? [O = 16, H = 1]
- A. 10.0s
- B. 12.5s
- C. 17.7s
- D. 32.0s
Calculate the amount in moles of a gas which occupies 10.5 dm3 at 6 atm and 30oC [R = 0.082 atm dm3 K-1 mol-1]
- A. 2.536
- B. 1.623
- C. 4.736
- D. 0.394
Ethene is prepared industrially by
- A. Reforming
- B. Polymerization
- C. Distillation
- D. Cracking
A particle that contains 9 protons, 10 neutrons and 10 electrons is
- A. positive ion
- B. neutral atom of a metal
- C. neutral atom of a non-metal
- D. negative ion
In the laboratory preparation of trioxonitrate (V) acid the nitrogen(iv) oxide formed as a by-product is removed by
- A. further heating
- B. adding concentrated H2SO4
- C. cooling the acid solution with cold water
- D. bubbling air through the acid solution
The gas that can be collected by downward displacement of air is
- A. chlorine
- B. sulphur (IV) oxide
- C. carbon (IV) oxide
- D. ammonia
An oxide XO2 has a vapour density of 32. What is the atomic mass of X?
- A. 20
- B. 32
- C. 14
- D. 12
According to Charle’s law, the volume of a gas becomes zero at
- A. −100°c
- B. −273°c
- C. −373°c
- D. 0°c
The carbon atoms on ethane are
- A. sp2 hybridized
- B. sp3 hybridized
- C. sp4 hybridized
- D. sp hybridized
The densities of two gases, X and Y are 0.5gdm-3 and 2.0gdm-3 respectively. What is the rate of diffusion of X relative to Y?
- A. 0.1
- B. 0.5
- C. 2.0
- D. 4.0
The reddish–brown rust on iron roofing sheets consists of
- A. Fe3 + (H2O)6
- B. FeO.H2O
- C. Fe2O3.XH2O
- D. Fe3O4.2H22O
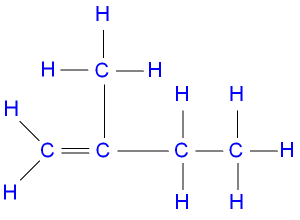
The IUPAC nomenclature of the structure above is
- A. 3-methybut-3-ene
- B. 2-methylbut-1-ene
- C. 2-ethylprop-1-ene
- D. 2-methylbut-2-ene
Calculate the mass of copper deposited when a current of 0.5 ampere was passed through a solution of copper(II) chloride for 45 minutes in an electrolytic cell. [Cu = 64, F = 96500Cmol-1]
- A. 0.300g
- B. 0.250g
- C. 0.2242g
- D. 0.448g
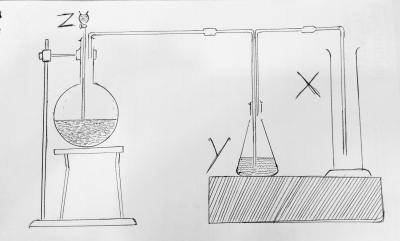
The diagram above. Y is
- A. fused CaO
- B. H2O
- C. NaOH
- D. Concentrated H2SO4
Cu2S(g) + O2(g) → 2Cu + SO2(g)
What is the change in the oxidation number of copper in the reaction?
- A. 0 to + 2
- B. 0 to + 1
- C. + 1 to 0
- D. + 2 to + 1