Which of the following metals cannot replace hydrogen from water or steam?
- A. Sodium
- B. Magnesium
- C. Iron
- D. Copper
The conductivity of an acid solution depends on the
- A. amount of ions present and their mobilities
- B. mobilities of the ions present
- C. amount of ions present
- D. temperature of the solution
Which of these sources of water may likely contain the least concentration of Ca\(^{2+}\) and Mg\(^{2+}\)?
- A. Spring water
- B. River water
- C. Tap water
- D. Sea water
Aluminium does not react with either dilute or concentrated trioxonitrate (V) acid because
- A. it is insoluble in either dilute or concentrated trioxonitrate (V) acid
- B. it is lower than hydrogen in the electrochemical series
- C. the reaction with trioxonitrate (V) acid has a light activation energy
- D. an insoluble oxide is formed on its surface which renders it passive
The presence of ammonia gas in a desiccator can exclusively be removed by
- A. phosphorus (V) oxide
- B. fused calcium chloride
- C. calcium oxide
- D. concentrated tetraoxosulphate (VI) acid
The collision theory explains reaction rates in terms of
- A. size of the product
- B. frequency of collision of the reactants
- C. size of reactant
- D. frequency of collision of the products
The end products of burning a candle in the atmosphere are water and
- A. carbon (II) oxide
- B. sulphur (IV) oxide
- C. carbon (IV) oxide
- D. sulphur (VI) oxide
2SO\(_2\) \(_{(g) }\)+ O\(_2\) \(_{(g) }\) ↔ 2SO\(_3\)\(_{(g) }\) ΔH = -395.7kJmol\(^{-1}\)
In the equation, an increase in temperature will shift the equilibrium position to the
- A. left and equilibrium constant decreases
- B. left and equilibrium constant increases
- C. right and equilibrium constant increases
- D. right and equilibrium constant decreases
The hydrogen ion concentration of a sample of orange juice is 2.0 X 10\(^{-11}\)moldm\(^{-3}\). What is its p\(^{OH}\)? [log102 = 0.3010]
- A. 3.30
- B. 13.00
- C. 10.70
- D. 11.30
An element X forms the following compounds with chlorine; XCl\(_4\), XCl\(_3\), XCl\(_2\). This illustrates the
- A. law of multiple proportions
- B. law of chemical proportions
- C. law of simple proportions
- D. law of conservation of mass
The knowledge of half-life can be used to
- A. Create an element
- B. Detect an element
- C. to combine elements
- D. Irradiate an element
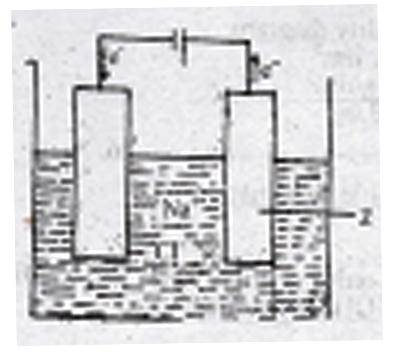
The figure above shows the electrolysis of molten sodium chloride. Z is the
- A. anode where the Cl\(^-\) ions are oxidized
- B. cathode where the Cl\(^-\) ions are reduced
- C. anode where the Na\(^+\) ions are reduced
- D. cathode where the Na\(^+\) ions are oxidized
Which of the following produces relatively few ions in solution?
- A. NaOH
- B. Ca(OH)\(_2\)
- C. KOH
- D. AI(OH)\(_3\)
The choice of method for extracting a metal from its ores depends on the
- A. strength of the core
- B. position of the metal in the electrochemical series
- C. source of the core
- D. position of the metal in the periodic table
Calculate the pH of 0.05 moldm\(^{-3}\) H\(_2\)SO\(_4\)
- A. 1.30
- B. 1.12
- C. 1.00
- D. 0.30
What mass of Cu would be produced by the cathodic reduction of Cu\(^{2+}\) when 1.60A of current passes through a solution of CuSO\(_4\) for 1 hour. (F=96500Cmol\(^{-1}\) , Cu=64)
- A. 7.64g
- B. 3.82g
- C. 1.91g
- D. 0.96g
The solubility of the solids that dissolves in a given solvent with the liberation of heat will
- A. increase with an increase in temperature
- B. decrease with an increase in temperature
- C. decrease with a decrease in temperature
- D. not be affected by changes in temperature
The constituent common to duralumin and alnico is
- A. Co
- B. Mn
- C. Al
- D. Mg
The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as;
- A. saponification
- B. dehydration
- C. esterification
- D. hydrolysis
Diamond is a bad conductor of electricity because its bonding electrons are used in
- A. crystal lattice formation
- B. covalent bond formation
- C. metallic bond formation
- D. coordinate bond formation
To what volume must 300cm\(^3\) of 0.60M sodium hydroxide solution be diluted to give a 0.40M solution?
- A. 450cm\(^3\)
- B. 300cm\(^3\)
- C. 200cm\(^3\)
- D. 150cm\(^3\)


