In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid?
- A. 5cm\(^3\)
- B. 10cm\(^3\)
- C. 20cm\(^3\)
- D. 50cm\(^3\)
Which of the following is used to power steam engines?
- A. lubricating oil
- B. coal
- C. bitumen
- D. diesel oil
2KClO\(_3\)(s) → 2KCl(s) + 3O\(_2\)(g)
The importance of the catalyst in the reaction above is that
- A. heating may not be required before the reaction takes place
- B. the reaction is controllable even at a high temperature
- C. the reaction produces large quantity of oxygen
- D. the reaction takes place more rapidly at a lower temperature
In the laboratory preparation of oxygen, the gas cannot be collected by downward displacement of air because
- A. the density of oxygen is greater than that of air
- B. the density of air is nearly the same as that of oxygen
- C. oxygen is a component of air
- D. air is an impure substance
When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are;
- A. nitrogen and carbondioxide
- B. the rare gases
- C. nitrogen and oxygen
- D. nitrogen and the rare gases
A balanced chemical equation obeys the law of
- A. conservation of mass
- B. definite proportions
- C. multiple proportions
- D. conservation of energy
Which of the following are mixtures?
I. Petroleum
II. Rubber latex
III. Vulcanizer’s solution
IV. Carbon sulphide
- A. I, II and III
- B. I, II and IV
- C. I and II only
- D. I and IV
The refreshing and characteristic taste of soda water and other soft drinks is as a result of the presence of
- A. carbon(IV)oxide
- B. carbon(ll)oxide
- C. soda
- D. glucose
The salt that reacts with dilute hydrochloric acid to produce a pungent smelling gas which decolourizes acidified purple potassium tetraoxomanganate (VII) solution is
- A. Na\(_2\)SO\(_4\)
- B. Na\(_2\)SO\(_3\)
- C. Na\(_2\)S
- D. Na\(_2\)CO\(_3\)
(I). 3CuO(s) + 2NH3(g) —–> 3Cu(s) + 3H2O(l) + N2(g)
(II). 2NH3(g) + 3Cl2(g) —–> 6HCl(g) + N2(g)
(III). 4NH3(g) + 3O2(g) —–> 6H2O(l) + N2(g)
The reactions represented by the equations above demonstrate the
- A. basic properties of ammonia
- B. acidic properties of ammonia
- C. reducing properties of ammonia
- D. oxidizing properties of ammonia
mE + nF —–> pG + qH
In the equation shown, the equilibrium constant is given by?
- A. \(\frac{[E]^m[F]}{[G]^p[H]^q}\)
- B. \(\frac{[E][F]}{[G][H]}\)
- C. \(\frac{[G]^p[H]^q}{[E]^m[F]^n}\)
- D. \(\frac{[G][H]}{[E][F]}\)
Beryllium and Aluminium have similar properties because they
- A. are both metals
- B. belong to the same group
- C. belong to the same period
- D. are positioned diagonally to each other
The radio isotope used in industrial radiography for the rapid checking of faults in welds and casting is?
- A. carbon-14
- B. phosphorus-32
- C. cobalt-60
- D. iodine-131
How many atoms are present in 6.0g of magnesium? [Mg = 24, N.A = 6.02 x 10 \(^{23}\)mol]
- A. 1.20 x 10\(^{22}\)
- B. 2.4 x 10\(^{22}\)
- C. 1.51 x 10\(^{23}\)
- D. 3.02 x 10\(^{22}\)
What is the concentration of a solution containing 2g of NaOH in 100cm3 of solution? [Na = 23, O =16, H = 1]
- A. 0.40 moldm\(^{-3}\)
- B. 0.50 moldm\(^{-3}\)
- C. 0.05 moldm\(^{-3}\)
- D. 0.30 moldm\(^{-3}\)
An aqueous solution of a metal salt, M. gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia Therefore the cation in M is
- A. Zn
- B. Ca
- C. Al
- D. Pb
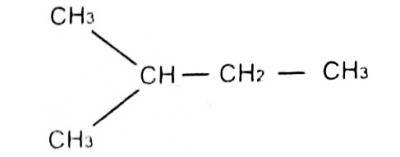
The lUPAC name for
- A. 1-methyl pentane
- B. 3-methylbutane
- C. 2-methylbutane
- D. 1-dimethyl propane
If one of the following oxides is heated with hydrogen or carbon using a bunsen burner. it is not reduced to the metal, Which one is it?
- A. lead oxide
- B. Magnesium oxide
- C. Copper oxide
- D. Tin oxide
What volume of oxygen will remain after reacting 8cm\(^3\) of hydrogen gas with 20cm\(^3\) of oxygen gas
- A. 10cm\(^3\)
- B. 12cm\(^3\)
- C. 14cm\(^3\)
- D. 16cm\(^3\)
The number of electrons in the valence shell of an element of atomic number 14 is?
- A. 1
- B. 2
- C. 3
- D. 4
Suitable reagents for the laboratory preparation of nitrogen are
- A. sodium dioxonitrate(III) and ammonium chloride
- B. sodium trioxonitrate(V) and ammonium chloride
- C. sodium chloride and ammonium trioxonitrate(V)
- D. sodium chloride and ammonium di-ozonitrate(III)


