A quantity of electricity liberates 3.6g of Silver from its salt. What mass of aluminium Will be liberated from its salt by the same quantity of electricity? [Al = 27, Ag = 108].
- A. 2.7g
- B. 1.2g
- C. 0.9g
- D. 0.3g
Methanoic acid mixes with water in all proportions and has about the same boiling point as water. Which of the following methods would you adopt to obtain pure water from a mixture of Sand, water and methanoic acid?
- A. Extractive distillation
- B. Filtration followed by distillation
- C. Neutralization with sodium hydroxide followed by distillation
- D. Neutralization with sodium hydroxide followed by filtration
In the preparation of oxygen by heating KCIO\(_3\) , in the presence of MnO\(_2\) only moderate heat is needed because the catalyst acts by \(_2\)
- A. lowering the pressure of the reaction
- B. increasing the surface area of the reaction
- C. increasing the rate of the reaction
- D. increasing the energy barrier of the reaction
Which of the following pairs of substances will react further with oxygen to form a higher oxide?
- A. CO\(_2\) and H\(_2\)O
- B. NO and H\(_2\)O
- C. CO and CO\(_2\)
- D. SO\(_2\) and NO
The boiling of fat and aqueous caustic soda is referred to as
- A. hydrolysis
- B. esterification
- C. acidification
- D. saponification
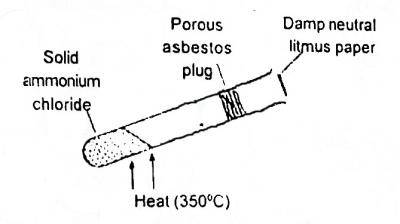
In the shown experiment (Fig. 1) the litmus paper will initially
- A. be bleached
- B. turn green
- C. turn red
- D. turn blue
If 1 litre of 2.2M sulphuric acid is poured into a bucket containing 10 litres of water and the resulting solution mixed thoroughly, the resulting sulphuric acid concentration will be
- A. 2.2M
- B. 1.1M
- C. 0.2M
- D. 0.11M
The periodic classification is an arrangement of the elements
- A. atomic weights
- B. isotopic weights
- C. molecular weights
- D. atomic numbers
Which of the following statements is correct about the periodic table?
- A. Elements in the same period have the same number of valence electrons
- B. The valence electrons of the elements in the same period increase progressively across the period
- C. Elements in the same group have the same number of electron shells
- D. The non-metallic Properties of the elements tend to decrease across each period
On which of the following is the solubility of a gaseous substance dependent?
I. Nature of solvent
II. Nature of solute
III. Temperature
IV. Pressure
- A. I, II, III and IV
- B. I and II only
- C. II only
- D. III and IV only
A basic postulate of the kinetic theory of gases is that the molecules of a gas move in straight lines between collisions. This implies that
- A. collisions are perfectly elastic
- B. forces of repulsion exist
- C. forces of repulsion and attraction are in equilibrium
- D. collisions are inelastic
A correct electrochemical series can be obtained from Na, Ca, Al, Mg, Zn, Fe, Pb, H, Cu, Hg, Ag, Au by interchanging
- A. Al and Mg
- B. Zn and Fe
- C. Zn and Pb
- D. Pb and H
The Consecutive members of an alkane homologous series differ by
- A. CH
- B. CH\(_2\)
- C. CH\(_3\)
- D. C\(_n\)H\(_n\)
Which of the following gases may not be dried with concentrated sulphuric acid?
- A. HCl\(_{(g)}\)
- B. NH\(_3\)
- C. Cl\(_2\)
- D. SO\(_2\)
An element used in the production of matches is
- A. nitrogen
- B. aluminium
- C. copper
- D. sulphur
According to the Kinetic Theory an increase in temperature causes the kinetic energy of particles to
- A. decrease
- B. increase
- C. be zero
- D. remain constant
To what temperature must a gas at 273k be heated in order to double both its volume and pressure?
- A. 298K
- B. 546K
- C. 819K
- D. 1092K
Which of the following is an example of a chemical change?
- A. dissolution of salt in water
- B. rusting of iron
- C. melting of ice
- D. separating a mixture by distillation
The type of bonding in [Cu(NH\(_3\))\(^4\)]\(^{2+}\) is
- A. coordinate
- B. electrovalent
- C. metallic
- D. covalent
What is the PH of 0.00 1 moldm\(^3\) solution of the sodium hydroxide
- A. 14
- B. 13
- C. 12
- D. 11
The Sulphide which is insoluble in dilute hydrochloric acid is
- A. FeS
- B. CuS
- C. ZnS
- D. Na2S


